![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
66 Cards in this Set
- Front
- Back
|
What pts would have a normal positive nitrogen balance where Nitrogen in > nitrogen out?
|
1. Growing children
2. Pregnancies 3. Convalescense |
|
|
What pts would have a normal negative nitrogen balance where Nitrogen in < nitrogen out?
|
1. following tissue destruction by trauma and injury (b/c you are degrading tissues)
2. Ilness related tissue breakdown 3. Protein deficient diet (b/c you are breaking down proteins for fuel.) |
|
|
What would a diet lacking or deficient in an essential amino acid lead to?
|
A negative nitrogen balance.
|
|
|
What is the overall goal of amino acid metabolism?
|
1. metabolized to avoid buildup of waste
2. amino acids are utilized as building blocks for protein synthesis 3. amino acids are utilized as precursors for the synthesis of numerous important metabolites. *amino acid metabolism is not focused on harnessing energy. |
|
|
How is most dietary carbon excreted?
|
As CO2
|
|
|
How is most dietary oxygen excreted?
|
As CO2 or water
|
|
|
What is the way humans excrete nitrogen waste?
|
As Urea, which is nontoxic and does not require much energy to form.
|
|
|
What does net formation of TCA metabolites lead to?
|
Gluconeogenesis
|
|
|
What does net removal of TCA metabolites lead to?
|
Not tolerated very well b/c OAA is necessary to allow turning of the TCA cycle.
|
|
|
What does transamination to form aspartate require?
|
Requires conversion of aspartate back to OAA while the production of ammonia is toxic. SO the urea cycle solves this problem
|
|
|
Where do the majority of transamination reactions occur?
|
In the liver
|
|
|
What are ketogenic amino acids?
|
Amino acids that are used to make acetyl CoA or acetoacetate.
|
|
|
What are glucogenic amino acids?
|
Amino acids that are used to make glucose, so anything that is used to make pyruvate, fumarate, OAA, alphaketoglutarate or succinyl CoA.
|
|
|
What amino acids are both gluconeogenic and ketogenic?
|
Tyrosine, tryptophan, phenylalanine, isoleucine, threonine
|
|
|
What enzyme deficiency cauces tyrosinemia type I? what accumulates? what is the treatment? what product is deficient?
|
Deficiency: fumaryl-acetoacetate hydrolase
Accumulation: fumarylacetoacetate and its metabolites particularly succinyl-acetone Treatment: dietary restriction of Phenylalanine and tyrosine Product D: Fumarate |
|
|
What is the deficiency of methylmalonyl CoA mutase deficiency? what accumulates? what product is deficient?
|
E. Deficiency: methylmalonyl CoA Mutase
Accumulation: methylmalonyl CoA Deficiency: Succinyl CoA |
|
|
What deficiency causes histidinemia? what accumulates? what is deficient?
|
E. Deficiency: histidase
Accumulation: histidine deficiency: urocanate/glutamate |
|
|
What enzyme deficiency causes Maple Syrup Urine Disease? what accumulates?
|
E. Deficiency: branched chain Alpha keto acid dehydrogenase
Accumulation: branched chain amino acids Valine, Isoleucine and leucine |
|
|
What enzyme deficiency causes cystathioninuria? what is deficient? what accumulates?
|
Enzyme: cystathionase
Accumulation: cystathionine and metabolites Product deficiency: cysteine and alphaketobutyrate/succinyl CoA. |
|
|
What enzyme deficiency causes homocystinuria? what accumulates? what is deficient?
|
Enzyme: cystathionine synthase
Accumulation: homocysteine and methionine Deficiency: alphaketobutyrate and succinyl CoA |
|
|
What are the fuels of the urea cycle?
|
Ammonia and aspartate
|
|
|
Where does the urea cycle predominantly occur?
|
W/in the liver
|
|
|
Where is carbamoyl phosphate synthase I located?
|
Primarily in the mitochondrial matrix
|
|
|
What urea cycle intermediates require a transporter to cross the innermitochondrial membrane?
|
Ornithine and citrulline
|
|
|
What is the first reaction of the urea cycle?
|
2ATP + HCO3 + NH3 to form carbamoyl phosphate
|
|
|
What is carbamoyl phosphate combined with? what do they form?
|
Ornithine to form citrulline
|
|
|
What amino acid does citrulline require to form arginino-succinate?
|
Aspartate and ATP
|
|
|
What TCA cycle intermediate is produced by the urea cycle? where does it come off?
|
Fumarate, comes off when arginino-succinate is turned into arginine
|
|
|
Where is urea given off in the urea cycle?
|
Last step, where arginine is converted to ornithine
|
|
|
How much ATP does the urea cycle require?
|
3 x ATP; but actually less is required in a way b/c fumarate feeds into the TCA which can be used to make ATP too.
|
|

What do ORC1 and ORC2 do?
|
Transport ornithine and citrulline across the inner mitochondrial membrane.
|
|
|
What are the two end product possibilities of transamination reactions?
|
Ammonia and Aspartate
|
|
|
What is the net energy cost to form Urea?
|
3 x ATP are required, but 2 x ATP are formed by fumarate feeding into the TCA and oxidative phosphorylation
SO 1 net ATP is required/lost |
|
|
What does mitochondiral carbamoyl phosphate synthetase I use as its nitrogen donor?
|
NH3
|
|
|
What does cytosolic carbamoyl phosphate synthetase II use as its nitrogen donor?
|
Glutamine
|
|
|
What is the job of cytosolic carbamoylphosphate synthetase II?
|
Primarily involved in pyrimidine synthesis
|
|
|
What is the Krebs bicycle?
|
Argininosuccinate forms fumarate when it is converted to arginine, fumarate (a TCA intermediate) can be turned into malate into OAA. OAA can be converted into aspartate using glutamate transamination. Asparate re-feeds into the urea cycle when it combines w/ citrulline to form argininosuccinate.
|
|
|
What are the two ways the amine group can feed into the urea cycle?
|
It is combined w/ a alpha-keto acid and alpha-ketoglutarate to form glutamate. THEN glutamate can either combine w/ NAD(P)+ to form ammonia OR it can combine w/ OAA to form aspartate.
IF IT FORMS NH3 it will combine w/ HCO3 and 2ATP to form carbamoyl phosphate IF IT FORMS ASPARTATE it will combine w/ 1 ATP and citrulline to form arginino-succinate. |
|
|
What is the role of the kidneys in metabolism of amino acids?
|
They take up glutamine and metabolize to ammonia for use in pH balance.
|
|
|
What do the kidneys do w/ citrulline?
|
They convert it to arginine and excrete it into the bloodstream
|
|
|
What do the kidneys do w/ phenylalanine?
|
They convert it to tyrosine and glycine and then to serine
|
|
|
What amino acid is required to make nitric oxide?
|
Arginine
|
|
|
Where is nNOS found?
|
In the brain
|
|
|
Where is iNOS found?
|
In macrophages/immune system.
|
|
|
What urea cycle enzymes allow efficient NO production? where are these enzymes found?
|
1. Arginosuccinic acid synthase
2. Arginosuccinase *Found in tissues that have to require lots of NO, like fibroblasts |
|
|
What enzyme deficiency could cause chronic inflammation, chromatin damage and eventual cancer?
|
iNOS and eNOS
|
|
|
What enzyme deficiency could lead to neurologic diseases such as parkinsons?
|
nNOS
|
|
|
What enzyme activity limits NO production?
|
arginase
|
|
|
What cells would need to import arginine and export citrulline?
|
Tissues deficient in arginosuccinic acid synthase and arginosuccinase (basically those tissues that cannot convert citrulline to arginine in order to generate NO)
|
|
|
So if you have a pt w/ kidney failure what urea cycle intermediate would they be deficient in? what would be a consequence of this?
|
They would be deficient in arginine, and a consequence of this is that they cannot produce NO and so they have bad vascular tension.
|
|
|
What does glutamine synthetase do?
|
Detoxifies ammonia in peripheral tissues by incorporating it into glutamine.
The glutamine is carried by the blood to the Liver where it is deaminated to regenerate NH4, which is incorporated into urea for excretion. |
|
|
Where is glutamine synthetase particularly important?
|
IN the brain and muscle
|
|
|
Where is glutaminase located?
|
In the liver and kidney
|
|
|
What does glutamine combined w/ water form?
|
Glutamate and NH3
|
|
|
Where is pyruvate conversion to alanine as a method of removing nitrogen an important part of metabolism?
|
In the sketetal muscle
|
|
|
What is the glucose-alanine cycle?
|
Occurs in skeletal muscle, it is where glucose is converted to pyruvate, which in turn is converted to alanine and alpha-ketoglutarate, the alanine travels in the circulatory system to the liver where it reforms pyruvate and glutamate.
The point is to transfer ammonia as alanine, instead of letting ammonia just travel in the bloodstream and be toxic. |
|
|
What nitrogen disposal methods can happen in muscle?
|
Glucose-alanine cycle and glutamine
|
|
|
What nitrogen disposal methods can happen in the intestines?
|
Glutamine can be converted to NH4 or alanine or citrulline
|
|
|
What nitrogen disposal methods can the kidneys use?
|
Glutamine or citrulline to arginine
|
|
|
IF you see high levels of alanine or ammonia or glutamine what does that mean?
|
IS there a large breakdown of protein occurring.
|
|
|
What are the Sy of urea cycle enzymes?
|
1. accumulation of glutamine and ammonia
-> causes hypothermia and apnea due to high ammonia 2. brain swelling/swollen astrocytes 3. lethargy and behavior abnormalities in childhood. |
|
|
What are the Sy of arginase deficiency?
|
1. Progressive spastic quadriplegia and mental retardation
2. hyperammonemia less than other urea cycle deficiencies |
|
|
What is the inheritance pattern of OTCD (ornithine transcarbamoylase deficiency)?
|
X-linked disorder.
|
|

|

|
|
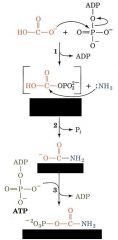
What enzyme carries out this reaction sequence?
|
Carbamoyl Phosphate Synthetase II
|
|
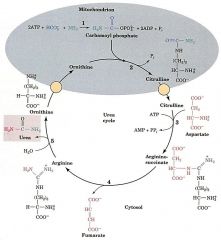
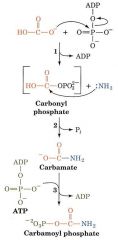
What enzyme catalyzes each step?
|
1. Carbamoyl phosphate synthetase
2. Ornithine Transcarbamoylase 3. Arginosuccinic acid synthase 4. Arginosuccinase 5. arginase |

