![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
Alanine
Ala A |
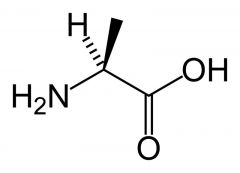
Aliphatic
No reactive groups Non-polar Interact favourably with other non-polar groups. |
|
|
Arginine
|
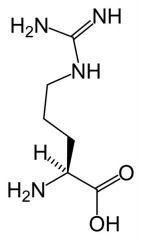
Capped with guanidino group.
group - planar stabilised by resonance. -Charge is delocalised over whole group. -ionised at physiological pH to NH-C-(NH2)2 + |
|
|
Asparagine
Asn N |

Not very chemically reactive
Polar H bond donor and acceptor amide is planar (partial double) |
|
|
Aspartate (Aspartic acid)
|

Completely ionised, negative charged and exist as resonance @ physiological pH
Very polar Often act as chelators of metal ions eg Calcium |
|
|
Cysteine
Cys C |
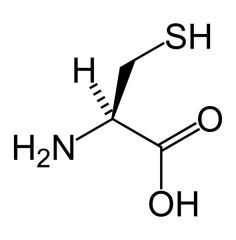
Non polar/polar
Very reactive in depronated form = use in active sites. C-SH group = sulfhydral |
|
|
Glutamate (Glutamic acid)
|
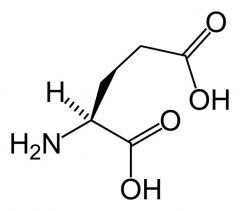
Completely ionised, negative charged and exist as resonance @ physiological pH
Very polar Often act as chelators of metal ions eg Calcium |
|
|
Glutamine
Gln Q |
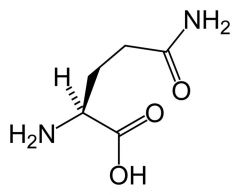
Not very chemically reactive
Polar H bond donor and acceptor amide is planar (partial double) |
|
|
Glycine
|
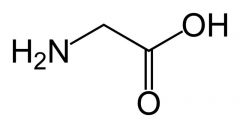
Aliphatic
No reactive groups Non-polar Much less restriction on phi and psi (steric reasons) and so make left and right handed turns in proteins. |
|
|
Histidine
HIS H |
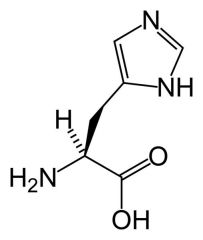
Imidazole ring- H acceptor(nucleophile) or donor(electrophile)
Therefore highly used in enzyme active sites. Hydrogen can be on either N. It is an electrophile when both Nitrogens have Hydrogens --> charged (+1) and also resonance with charged form of HIS (refer to lec) Nitrogen without H has lone pair on outside and has the double bond. |
|
|
Isoleucine
|
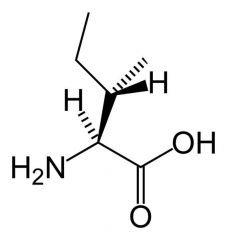
Aliphatic
No reactive groups Non-polar Interact favourably with other non-polar groups. extra chiral centre |
|
|
Leucine
|
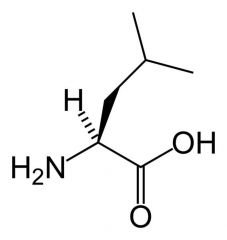
Aliphatic
No reactive groups Non-polar Interact favourably with other non-polar groups. |
|
|
Lysine
|
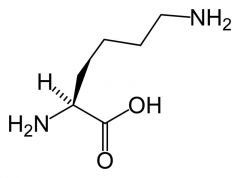
Long aliphatic side chain.
Amine cap - -very polar and almost completely ionised to C-NH3+ --> thus positively charged. |
|
|
Methionine
Met M |
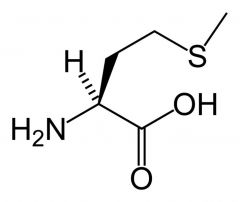
Non-polar
Not Particularly reactive |
|
|
Phenylalanine
Phe F |
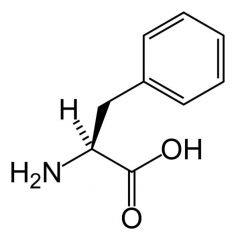
Non-polar
Unreactive Absorbs a little UV light. |
|
|
Proline
Pro P |
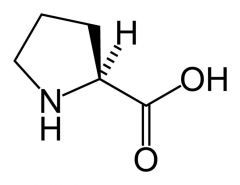
Aliphatic
No reactive groups Non-polar Interact favourably with other non-polar groups. No NH group: imino acid So can't act as backbone H-bond donor Very limited rotation so restricted Phi to ~ -60 peptide bond usually trans 1:1000 but 1:4 for X-Pro bond. |
|
|
Serine
Ser S |
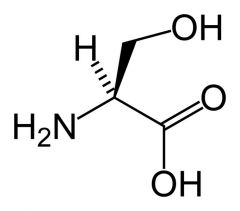
Not very chemically reactive.
H-bond donor or acceptor (OH --> OH2 or O) Polar |
|
|
Threonine
Thr T |

Not very chemically reactive.
H-bond donor or acceptor (OH --> OH2 or O) Polar extra chiral centre |
|
|
Tryptophan
Trp W |
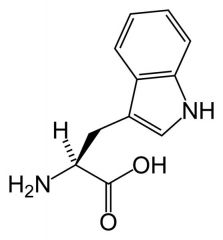
Has Indole ring(first ring)
Largely non-polar Rarest side chain Electron Rich = interact with electron poor like arginine Absorbs UV light and is fluorescent. Spectral properties are sensitive to immediate surroundings = can tell if buried or exposed in a protein. |
|
|
Tyrosine
Tyr Y |
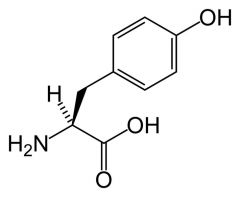
Largely non-polar except for OH
OH can hydrogen bond hydroxyl group has pKa ~10 Absorbs UV light and fluorescent Spectral properties are sensitive to immediate surroundings = can tell if buried or exposed in a protein. |
|
|
Valine
Val V |
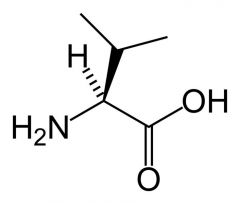
Aliphatic
No reactive groups Non-polar Interact favourably with other non-polar groups. beta branched - branched at beta carbon. |

