![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
60 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What are some elements from the periodic table?
|

|
|
|
|
Whats ionic bonding?
|
•metal and non-metals
•metals loose electrons and become positive ions, non-metals gain electrons to form negative ions. |

|
|
|
Whats the limestone cycle?
|

|
|
|
|
Explain fractional distillation of crude oil?
|
The fractionating column works continuously with heated crude oil piped in the bottom. The vaporised oil rises up the column and the various fractions are constantly tapped off at the different levels where they condense.
|

|
|
|
Why cant you use pure iron?
|
•the pure iron has a regular arrangement of identical atoms
•the layers of atoms can slide past each other •making the iron soft and too bendy for most uses |
|
|
|
Why cant you use pure iron?
|
•the pure iron has a regular arrangement of identical atoms
•the layers of atoms can slide past each other •making the iron soft and too bendy for most uses |
|
|
|
Why are alloys harder than pure metals?
|
•different elements have different sized atoms.
•so when they are mixed together they will upset the layers of pure iron atoms •making it more difficult for then to slide over each other •so alloys are harder |
|
|
|
Why cant you use pure iron?
|
•the pure iron has a regular arrangement of identical atoms
•the layers of atoms can slide past each other •making the iron soft and too bendy for most uses |
|
|
|
Why are alloys harder than pure metals?
|
•different elements have different sized atoms.
•so when they are mixed together they will upset the layers of pure iron atoms •making it more difficult for then to slide over each other •so alloys are harder |
|
|
|
What are the first 4 alkanes?
|

|
|
|
|
Why cant you use pure iron?
|
•the pure iron has a regular arrangement of identical atoms
•the layers of atoms can slide past each other •making the iron soft and too bendy for most uses |
|
|
|
Why are alloys harder than pure metals?
|
•different elements have different sized atoms.
•so when they are mixed together they will upset the layers of pure iron atoms •making it more difficult for then to slide over each other •so alloys are harder |
|
|
|
What are the first 4 alkanes?
|

|
|
|
|
Whats the alkane formula?
|
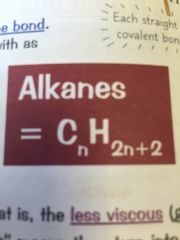
|
|
|
|
Why cant you use pure iron?
|
•the pure iron has a regular arrangement of identical atoms
•the layers of atoms can slide past each other •making the iron soft and too bendy for most uses |
|
|
|
Why are alloys harder than pure metals?
|
•different elements have different sized atoms.
•so when they are mixed together they will upset the layers of pure iron atoms •making it more difficult for then to slide over each other •so alloys are harder |
|
|
|
What are the first 4 alkanes?
|

|
|
|
|
Whats the alkane formula?
|

|
|
|
|
Whats the trend in shorter hydrocarbon molecules?
|
•the shorter the molecule the more runny -viscous-the hydrocarbon is
•the shorter the molecules the more volatile they are -means they turn into a gas at a lower temperature - and the lower the boiling point •the shorter the molecules, the more flammable the hydrocarbon is |
|
|
|
Why cant you use pure iron?
|
•the pure iron has a regular arrangement of identical atoms
•the layers of atoms can slide past each other •making the iron soft and too bendy for most uses |
|
|
|
Why are alloys harder than pure metals?
|
•different elements have different sized atoms.
•so when they are mixed together they will upset the layers of pure iron atoms •making it more difficult for then to slide over each other •so alloys are harder |
|
|
|
What are the first 4 alkanes?
|

|
|
|
|
Whats the alkane formula?
|

|
|
|
|
Whats the trend in shorter hydrocarbon molecules?
|
•the shorter the molecule the more runny -viscous-the hydrocarbon is
•the shorter the molecules the more volatile they are -means they turn into a gas at a lower temperature - and the lower the boiling point •the shorter the molecules, the more flammable the hydrocarbon is |
|
|
|
How is acid rain formed?
|
•sulphur dioxide is one of the gases that causes it
•when it mixes with clouds it forms dilute sulphuric acid, then falls as acid rain |

|
|
|
Why cant you use pure iron?
|
•the pure iron has a regular arrangement of identical atoms
•the layers of atoms can slide past each other •making the iron soft and too bendy for most uses |
|
|
|
Why are alloys harder than pure metals?
|
•different elements have different sized atoms.
•so when they are mixed together they will upset the layers of pure iron atoms •making it more difficult for then to slide over each other •so alloys are harder |
|
|
|
What are the first 4 alkanes?
|

|
|
|
|
Whats the alkane formula?
|

|
|
|
|
Whats the trend in shorter hydrocarbon molecules?
|
•the shorter the molecule the more runny -viscous-the hydrocarbon is
•the shorter the molecules the more volatile they are -means they turn into a gas at a lower temperature - and the lower the boiling point •the shorter the molecules, the more flammable the hydrocarbon is |
|
|
|
How is acid rain formed?
|
•sulphur dioxide is one of the gases that causes it
•when it mixes with clouds it forms dilute sulphuric acid, then falls as acid rain |

|
|
|
Why do we crack crude oil?
|
•long-chain hydrocarbons are thick and gloopy which isn't useful so they go through fractional distillation and are turned into smaller ones-cracking-
•some of the products are useful •it also produces ethene which is needed for making plastic |
|
|
|
Why cant you use pure iron?
|
•the pure iron has a regular arrangement of identical atoms
•the layers of atoms can slide past each other •making the iron soft and too bendy for most uses |
|
|
|
Why are alloys harder than pure metals?
|
•different elements have different sized atoms.
•so when they are mixed together they will upset the layers of pure iron atoms •making it more difficult for then to slide over each other •so alloys are harder |
|
|
|
What are the first 4 alkanes?
|

|
|
|
|
Whats the alkane formula?
|

|
|
|
|
Whats the trend in shorter hydrocarbon molecules?
|
•the shorter the molecule the more runny -viscous-the hydrocarbon is
•the shorter the molecules the more volatile they are -means they turn into a gas at a lower temperature - and the lower the boiling point •the shorter the molecules, the more flammable the hydrocarbon is |
|
|
|
How is acid rain formed?
|
•sulphur dioxide is one of the gases that causes it
•when it mixes with clouds it forms dilute sulphuric acid, then falls as acid rain |

|
|
|
Why do we crack crude oil?
|
•long-chain hydrocarbons are thick and gloopy which isn't useful so they are cracked
•some are useful as fuels •it also produces ethene which is needed for making plastic |
|
|
|
What is cracking?
|
•cracking is a thermal decomposition reaction- breaking molecules down by heating them
•heat the long chain hydrocarbon to vaporise it •the vapour is then passed over a powdered catalyst •temp 400-700 •aluminium oxide is the catalyst used •the molecules split apart-crack- on the surface |
|
|
|
Some products of cracking?
|

|
|
|
|
How can alkenes be used to produce polymers?
|
•polymerisation- means joining together lots of small alkene molecules(monomers) to form large molecules(polymers)
|

Many ethene molecules can be joined to produce poly(ethene)
|
|
|
What are the benefits of vegetable oils for cooking?
|
•vegetable oils have higher boiling points than water, this means they can cook foods at a higher temp and quicker
•gives food a different flavour •increases the amount of energy we get from eating it |
|
|
|
Some products of cracking?
|

|
|
|
|
How can alkenes be used to produce polymers?
|
•polymerisation- means joining together lots of small alkene molecules(monomers) to form large molecules(polymers)
|

Many ethene molecules can be joined to produce poly(ethene)
|
|
|
What are the benefits of vegetable oils for cooking?
|
•vegetable oils have higher boiling points than water, this means they can cook foods at a higher temp and quicker
•gives food a different flavour •increases the amount of energy we get from eating it |
|
|
|
Saturated or unsaturated oils
|
•unsaturated oils contain c=c double bonds
•oils and fats contain long-chain molecules •and unsaturated oil will decolourise bromine water •monounsaturated fats contain one c=c double bond •polyunsaturated fats contain more than one |
|
|
|
Some products of cracking?
|

|
|
|
|
How can alkenes be used to produce polymers?
|
•polymerisation- means joining together lots of small alkene molecules(monomers) to form large molecules(polymers)
|

Many ethene molecules can be joined to produce poly(ethene)
|
|
|
What are the benefits of vegetable oils for cooking?
|
•vegetable oils have higher boiling points than water, this means they can cook foods at a higher temp and quicker
•gives food a different flavour •increases the amount of energy we get from eating it |
|
|
|
Saturated or unsaturated oils
|
•unsaturated oils contain c=c double bonds
•oils and fats contain long-chain molecules •and unsaturated oil will decolourise bromine water •monounsaturated fats contain one c=c double bond •polyunsaturated fats contain more than one |
|
|
|
How to hydrogenate unsaturated oils?
|
•liquid at room temp
•they can be hardened by reacting them with hydrogen in the presence of a nickel catalyst at about 60 degrees •the hydrogen reacts with the double bonded carbons and opens double bonds |
|
|
|
Some products of cracking?
|

|
|
|
|
How can alkenes be used to produce polymers?
|
•polymerisation- means joining together lots of small alkene molecules(monomers) to form large molecules(polymers)
|

Many ethene molecules can be joined to produce poly(ethene)
|
|
|
What are the benefits of vegetable oils for cooking?
|
•vegetable oils have higher boiling points than water, this means they can cook foods at a higher temp and quicker
•gives food a different flavour •increases the amount of energy we get from eating it |
|
|
|
Saturated or unsaturated oils
|
•unsaturated oils contain c=c double bonds
•oils and fats contain long-chain molecules •and unsaturated oil will decolourise bromine water •monounsaturated fats contain one c=c double bond •polyunsaturated fats contain more than one |
|
|
|
How to hydrogenate unsaturated oils?
|
•liquid at room temp
•they can be hardened by reacting them with hydrogen in the presence of a nickel catalyst at about 60 degrees •the hydrogen reacts with the double bonded carbons and opens double bonds |
|
|
|
Hydrogenated oils benefits
|
•have higher melting points(good for spreads)
|
|
|
|
Whats an emulsion?
|
•you can mix oil and water to create an emulsion which are made up of droplets of one liquid suspended in another liquid.
|
|
|
|
Hydrophobic and hydrophillic
|

•Hydrophilic head likes water and hates oil
•Hydrophobic tail likes oil and hates water |
|

