![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
|
Hydration |
H2O -> H + OH Markov H2SO4 or H3PO4 H3O+ |
|
|
Hydrogenation |
H2 -> H + H
|
|
|
Dihydroxylation |
HOOH -> OH + OH |
|
|
Oxidative Cleavage |
O2 -> O + O (Two separate molecules) Split molecule in half, forms either ketones or aldehydes |
|
|
Epoxidation |
O in triangle Uses Peroxyacid MCPBA Uses H3O+ to open ring and form two OH's Attacks on backside |
|
|
Halogenation |
X2 -> X + X Anti-Addition Forms triangle, attacks from rear |
|
|
Halohydride Formation |
XOH -> X + OH Markov Anti-Addition X2 H2O |
|
|
HX Addition |
HX -> H + X Markov |
|
|
Cyclopropanation |
CH2 -> CH2 in triangle Diazomethane- CH3N2, UV or Heat Simmons-Smith- CH2I2 and ZnCu (Used) Alpha Elimination of a Haloform- CHX3, NaOH,H2O Retains cis or trans |
|
|
Free-Radical Initiation |
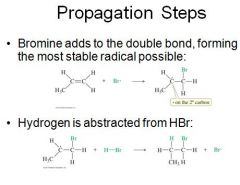
|
|
|
Oxymercuration-Demercuration Reaction |
Hydration using Mercury as intermediate Hg taken off be NaBH4 Hg forms triangle, H2O attacks from other side Markov |
|
|
Alkoxymercuraiton-Demercuration |
Same as last one, except ether instead of OH |
|
|
Hydroboration |
Hydration with BH3 intermediate BH3 THF H2O2 NaOH H2O Anti-Markov Syn Addition |
|
|
-OH |
Strong base promotes E2 reaction |
|
|
Catalytic Hydrogenation |
Hydrogenation with catalyst Pt, Pd, Ni Syn |
|
|
Syn Hydroxelation |
Adds 2 OHS on same side OsO4 and H2O2 KMnO4 and OH- |
|
|
KMnO4 |
Harsh Conditions- Oxidative Cleavage Mild Conditions- Syn Hydroxelation |
|
|
Ozonolysis |
O3 and (CH3)2S Forms ketones or aldehydes (CH3)2SO |
|
|
Cationic Polymerization |
Pronate Molecule, attach more to pronated side |
|
|
Radical Polymerization |
Forms radicals, then combines them |
|
|
Anionic Polymerization |
Creates anions, combines them |

