![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
Compound |
When two or more substances are chemically bond together |
|
|
Mixture |
When two or more substances are mixed together and can be easily seperated. seperated. seperated. |
|
|
Element |
An element is a substance made up of only one type of atom |
|
|
Oxygen Gas Test |
You put hydrogen peroxide and mangese powder (catalyst), and the product of the two reactants make oxygen and water. Light a stick and the blow it out, and put into the test tube, and the stick will glow more, or completely relight. O2 relights a glowing stick makes test tube smoky manganese fizzes up |
|
|
Hydrogen Gas Test |
Put metal and acid into test tube, cover mouth of test tube to collect hydrogen gas, light a stick up with flame, and put it in hydrogen, and the result off it will Make a pop sound. H2 explode when ignited Bottom of test tube heats up Mg fizzes |
|
|
Carbon Dioxide Test |
CO2 turns limewater milky colored HCl fizzes up Calcium Carbonate dissolves in acid |
|
|
Metal + Oxygen |
Metal oxide |
|
|
Metal + water |
Metal Hydroxide + hydrogen Gas |
|
|
Metal + Acid |
Salt + hydrogen gas |
|
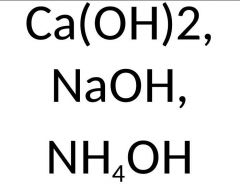
What are they and what do they have in common? |
Calcium Hydroxide, Sodium Hydroxide, Ammonium Hydroxide, They are all basic |
|
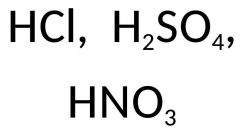
Name the formulas and what do they have in common? |
Hydrochloric Acid, Sulfuric Acid, Nitric Acid They are all acids |
|
|
Define neutralization |
The reaction between an acid and a base, and there is no excess OH- or H+ |
|
|
acid + base |
Water + Salt |
|
|
What is an Acid? |
A chemical which gives off hydrogen ions, when dissolved in water. |
|

odour - poor conductor of heat and electricity - has an odour- poor conductor of heat and electricity - doesn't break apart when hammered - are mostly matter or gases |
Non - metal |
|

- Shiny when freshly cut - can be reshaped - good conductors of heat and electricity |
Metals |
|
|
Sodium + water? |
Sodium Hydroxide + hydrogen gas |

