![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
103 Cards in this Set
- Front
- Back
|
Covalent Bonds |
Bonds b/t atoms w/ shared pairs of electrons |
|
|
How many Kilocalories does it take to break a covalent bond? |
80-100 Kilocalories |
|
|
Electronegativity |
The atoms nucleus' attraction force of electrons |
|
|
Polar |
Opposing charges on a molecule in their on region. |
|
|
Nonpolar |
Molecules w/ the same charge equally distributed around the molecule |
|
|
Anions |
Have a negative |
|
|
Cations |
Have lost electrons |
|
|
Free radicals |
are unstable atoms or molecules w/ unpaired electrons |
|
|
Superoxide dismutase (SOD) |
is an enzyme that destroy the superoxide radical. |
|
|
Noncovalent Bonds |
Don't depend on the sharing of electron w/ other atoms, but rather on attractive forces b/t atoms having an opposite charge. |
|
|
Ionic Bond |
Attraction b/t fully charged components EX. NaCl |
|
|
Hydrogen Bond (H-bonds) |
When covalently bound hydrogens have a partial (+) charge, due the electronegativity of the other atom, attracts electrons of a second atom. (O,N,&F) |
|
|
Van de Waals forces |
Attractions b/t nonpolar molecules, are due to transient dipole formation. |
|
|
Acids |
Release protons |
|
|
Bases |
Accept protons |
|
|
Amphoteric |
Molecules can act as either acids or bases |
|
|
pH |
pH = –log [H+] |
|
|
Biochemicals |
Carbon-containing molecules produced by living organisms |
|
|
Cholesteral |
A carbon chain w/ a hydroxly group |
|
|
Functional groups |
Groups of atoms giving organic molecules different characteristics and properties. |
|
|
What are the four major Macromolecules categories? |
Proteins Nucleic acids Polysaccharides Lipids |
|
|
Macromolecules |
Molecules that form the structure and carry out the activities of cells are huge, highly organized |
|
|
What are the two most common linkages b/t functional groups? |
Ester Bonds Amide Bonds |
|
|
Ester Bonds |
Form b/t carboxylic acids and alcohols |
|
|
Amide Bonds |
Form b/t carboxylic acids and amines |
|
|
Hydroxyl group |
(—OH) |
|
|
Carboxyl group |
(—COOH) |
|
|
Sulfhydryl group |
(—SH) |
|
|
Amino |
(-NHH) |
|
|
Metabolic Pathway |
A series of chemical reactions in the cell |
|
|
Metabolic Intermediates |
The compounds formed along the pathways leading to the end products, might have not functions per se. |
|
|
List the Molecules of Miscellaneous function: |
Vitamins Steroid/ A.A. hormones ATP Metabolic waste |
|
|
Vitamins |
Primarily as adjuncts to proteins |
|
|
Carbohydrates |
Glycans |
|
|
Carbohydrates |
Simple sugars (monosaccharides) and all larger molecules constructed of sugar Building building. Functions primarily as stores of chemical energy and building materials. |
|
|
Carbonyl groups |
(C=O) |
|
|
Glycosidic Bond |
A bond formed b/t the C1 atom of a sugarl and the hydroxyl group of another sugar. generating an ester linkage (-C-O-C-) |
|
|
Oligosaccharides |
Small carbohydrate chains. |
|
|
Where are Oligosaccharides found? |
Bound to cells surface proteins and lipids, and may be used for cell recognition |
|
|
Disaccharides |
A chain of two carbohydrates. |
|
|
What are disaccharides used for? |
Sources of readily available energy |
|
|
Polysaccharides |
are polymers of sugars joined by glycosidic bonds |
|
|
Glycogen |
is an animal product made of branched glucose polymers |
|
|
Starch |
is a plant product made of both branched and unbranched and glucose polymers |
|
|
Sucrose |
Composed of Glucose and Frutose joined by an Alpha (1 → 2) linkage |
|
|
Lactose |
Composed of Glucose and Galactose Joined by an Beta (1 → 4) linkage |
|
|
What are the Two types of Glycosidic bonds? |
Alpha (1 → 4) linkage: Type 2 Alpha (1 → 6) linkage: Type 1 |
|
|
Starch |
Is actually a mixture of two different polymers amylose and amylopectin |
|
|
Amylose |
is an unbranched, helical molecule whose sugars are joined by an Alpha (1 → 4) linkage |
|
|
Amylopectin |
Is like glycogen but much less branched and have an irregular branch patern |
|
|
Cellulose |
Unbranched polysaccharides whose sugars are joined by a Beta (1 → 4) linkage |
|
|
Chitin |
Unbranched polymer of the sugar N-acetylglucosamine. Found in invertebrate exoskeleton. |
|
|
N-Acetylglucosamine |
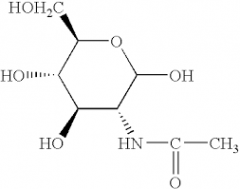
|
|
|
Glycosaminoglycans (GAG) |
Polysaccharides is a disaccharide chain(-A-B-A-B-) |
|
|
Triacylglycerol |
Glycerol molecule linked by ester bonds to three fatty Acids |
|
|
Triacylglycerol |

|
|
|
Fatty Acids |
Long unbranched hydrocarbon chains w/ a single carboxyl group at one end |
|
|
Lipids |
A diverse group of nonpolar molecules |
|
|
Steroids |
Four ringed hydrocarbon skeleton with a hydroxyl group |
|
|
Proteins |
Are A.A. polymers |
|
|
What are the parts of an A.A.? |
Alpha Carbon Amine group Carboxyl group Variable R group |
|
|
How are proteins made? |
By linking together A.A. by peptide bonds into unbranched polypeptide chain |
|
|
Peptide bond |
The linkage of a carboxyl group to the amino group of one A.A. to the another |
|
|
Polar Charged A.A. |
The R group contains a relatively strong organic acid or base. As a result they can form ionic bonds. |
|
|
Polar Uncharged A.A. |
The R group has partial charges and thus can form H-bonds |
|
|
Nonpolar A.A. |
The R group is hydrophobic and can not form electrostatic bonds. Are found tightly packed w/in the proteins. |
|
|
Glycine |
Lack of an R group allow for flexibility which allow it to form hinge like regions. |
|
|
Proline |
Is a hydrophobic, often products kinks or hinges |
|
|
Cystenie |
Contains a reactive sulfhydryl group, which form a disulfide bond w/ another csytenie in order to stabilize the intricate shape of proteins |
|
|
Disulfide bridge |
(—S—S—) |
|
|
What are the four levels of protein construction? |
Primary Structure Secondary Structure Tertiary Structure Quarternary Structure |
|
|
Primary structure |
The sequence of A.A. in the polymer, is critical to the protein function. |
|
|
Secondary structure |
The conformation of A.A. into Alpha helix, Beta sheets, hinges, turns, loops, or finger like extensions. Arrangement of hydrophobic and hydrophilic regions internally or externally |
|
|
Alpha helix |
the backbone of the polypeptide assumes the form of cylindrical, twisting spiral |
|
|
Tertiary Structure |
Conformation of the entire polypeptide, a series of noncolavent bonds b/t side chains |
|
|
X-ray crystallography |
The method use to figure out the Tertiary structure of proteins. By shooting a crystal of the protein with a x-ray the diffracted radiation hits a radiation sensitive plate, where they then use math to discern the structure that could cause the plate pattern. |
|
|
Fibrous proteins |
elongated shape |
|
|
Globular proteins |
Compact shape |
|
|
Myoglobin |
Has a heme prosthetic group that binds O2. The first to globular protein to be discern via X-ray Crystallography |
|
|
Quaternary Structure |
Refers to proteins and that are composed of subunits |
|
|
Multiprotein complex |
Different proteins, each w/ a specific function, become physically associated |
|
|
Pyruvate dehydrogenase |
Convert Pyruvate into Acetyl CoA |
|
|
Molecular Chaperones |
Helper protein that help unfolded or misfolded proteins achieve the proper folded pattern |
|
|
What are some conditions linked oxygen derived free radicals? |
Alzheimer, emphysema, cancer, diabetes, rheumatoid arthritis |
|
|
Antioxidants |
substances that inactivate oxygen derived free radicals. Ex: Glutathione, Selenium, Zinc, beta-carotene, vit. C and E |
|
|
Calorie restriction extends what? |
Lifespan, b/c there is a decrease in free radical production |
|
|
What modern use if there for chitin? |
Used to make a strong flexible surgical thread |
|
|
Atherosclerosis |
Plaque deposits |
|
|
Citrullination |
The conversion of the A.A. arginine in a protein into the A.A. citrulline |
|
|
Peptidylarginine deiminases (PADs) |
Replace the aldimine group (=NH) w/ a ketone group (=O) |
|
|
What does cirtullineation do? |
Control the expression of genes |
|
|
Posttranslational Modification (PTMs) |
Proteins the DNA doesn't code for, but are made via modification to existing A.A. |
|
|
How would one classify Citrilline |
A PTMs that is apart of the urea cycle |
|
|
What causes Sickle disease? |
The Valine replacement of Glutamic acid |
|
|
Selenocysteine |
A.A. found in prokaryotes and most eukaryotes, but is a PTMs |
|
|
Pyrrolysine |
A.A. found in some Archea and only one bacterium, but is a PTMs |
|
|
Beta pleated sheet |
Segment of polypeptide side by side; assume folds of pleated conformation |
|
|
How many A.A. are in one complete cylindrical twist of the Alpha helix? |
3.6 |
|
|
Domains |
Distinct modules, that independently fold. A functional region that catalysis independently and is found in other proteins. |
|
|
Ribonuclease |
protein that self assemble |
|
|
Hsp 70 family |
Bind emerging proteins and prevent inappropriate interactions |
|
|
Chaperonins |
allow large new proteins to assemble w/o interference from other macromolecules |
|
|
What causes Creutzfeld-Jakob Disease by? |
Miss folded proteins |

