![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
155 Cards in this Set
- Front
- Back
|
Proteins
|
Excellent immunogens, antigen presented to MHC class I- and MHC class II-restricted cells.
o T-lymphocyte-dependent antigens. o Immunizations which are protein based antigens include diphtheria and tetanus. |
|
|
Polysaccharides
|
T-lymphocyte-independent antigens.
o The meningococcal and 23 valent pneumococcal immunizations are examples of T lymphocyte independent antigens. |
|
|
Composite of antigens
|
Proteins, Polysaccharides, NA, and Lipids
|
|
|
Lipids
|
MHC-like CD1 molecules bind lipid antigens that are recognized by natural killer T lymphocytes (NKT cells), γδ T lymphocytes, and other lipid antigen-specific T lymphocytes.
|
|
|
Antigenic Determinants as recognized by B Lymphocytes
|
Linear determinants or tertiary structure. Carbohydrates, amino acids, and nucleic acids. Four to eight residues. Polysaccharides are considered T-lymphocyte-independent (TI) antigens.
|
|
|
Proteins
|
Excellent immunogens, antigen presented to MHC class I- and MHC class II-restricted cells.
o T-lymphocyte-dependent antigens. o Immunizations which are protein based antigens include diphtheria and tetanus. |
|
|
Polysaccharides
|
T-lymphocyte-independent antigens.
o The meningococcal and 23 valent pneumococcal immunizations are examples of T lymphocyte independent antigens. |
|
|
Composite of antigens
|
Proteins, Polysaccharides, NA, and Lipids
|
|
|
Lipids
|
MHC-like CD1 molecules bind lipid antigens that are recognized by natural killer T lymphocytes (NKT cells), γδ T lymphocytes, and other lipid antigen-specific T lymphocytes.
|
|
|
Antigenic Determinants as recognized by B Lymphocytes
|
Linear determinants or tertiary structure. Carbohydrates, amino acids, and nucleic acids. Four to eight residues. Polysaccharides are considered T-lymphocyte-independent (TI) antigens.
|
|
|
Superantigens bind to the Vbeta region of TCR and outside of the peptide binding group on MHC molecules.
|
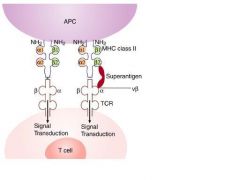
|
|
|
Hapten
|
Antigen that requires covalent linkage to a carrier to stimulate immune response (eg, penicillin is a hapten).
|
|
|
Carrier:
|
A macromolecular substance to which a hapten is coupled in order to produce an immune response against the hapten.
|
|
|
Adjuvant:
|
Molecules that enhance the immune response. Adjuvants release bound antigens to antigen-presenting cells (APCs) over a prolonged period, interact with toll-like receptors, and stimulate chemokine and cytokine release. Examples include the following: Alum. Freund’s adjuvant: emulsified bacterial products (eg, bacille Calmette-Guérin (BCGs]). Incomplete Freund’s adjuvant: water in oil emulsification. Ribi adjuvant system: Squalene-Tween80-water and oil emulsification. Titermax: copolymers polyoxypropylene (POP) and polyoxyethylene (POE).
|
|
|
Epitope (Antigenic determinant):
|
Antigenic component identified by a unique antibody. May represent linear amino acid sequence or antigenic tertiary structure.
|
|
|
Superantigen:
|
Antigens that activate a large number of polyclonal T lymphocytes. Examples of microbial toxin superantigens are provided
|
|
|
Superantigens binds to the ___ of the TCR
|
Superantigens bind to the Vβ chain of the T cell receptor (TCR) bypassing the need for the specific major histocompatibility complex (MHC), peptide, or TCR trimolecular complex required for signal 1 (Figure 1-1).
|
|
|
Antigenic Determinants recognized by B cells
|
Linear determinants or tertiary structure. Carbohydrates, amino acids, and nucleic acids. Four to eight residues. Polysaccharides are considered T-lymphocyte-independent (TI) antigens.
|
|
|
AD Recognized by T lymphocyte:
|
Linear determinants. Amino acid peptides. Eight to 30 amino acids (MHC class I 8-11 aa and MHC class II 10-30 aa).
|
|
|
MHC I are constitutively expressed by _____ and induced by ______
|
Most nucleated cells; IFN gamma
|
|
|
MHC 2 are constitutively expressed by _____ and induced by ______
|
APC (Dendritic cells, Macrophages,
and B lymphocytes), thymic epithelia, and activated T lymphocytes; IFN gamma |
|
|
Map of the MHC Genome; encoded on short arm Chr 6
|
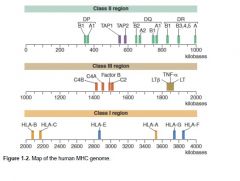
|
|
|
Class III region encodes for
|
Proteins of the complement system: Factor B, C4a, C4b, and C2. Cytokines: tumor necrosis factor (TNF)α, and lymphotoxins α and β. Heat shock proteins.
|
|
|
Class I encodes for
|
HLA E: NK cell recognition. HLA F: localized to endoplasmic reticulum and Golgi apparatus. HLA G: on fetal-derived placental cells. HLA H: involved in iron metabolism.
|
|
|
For MHC Class I, polypeptides remain sequestered in the endoplasmic reticulum by interacting with:
|
Calnexin, Calreticulin, Erp57, and Tapasin.
|
|
|
Organisms that enter the cytoplasm are degraded to antigenic peptides by the
|
proteasome:
o The proteasome is a multi-subunit proteinase. Four seven-membrane rings have catalytic subunits. o Examples of subunits are: low molecular mass polypeptide (LMP) 7 and LMP2. o LMPs are encoded in MHC class II locus. |
|
|
Antigenic peptides are transported into the endoplasmic reticulum by transporter associated with antigen processing (TAP) proteins.
|
Energy-dependent transport of peptides.
o Composed of two subunits: TAP1 and TAP2, both of which must be present for function. o TAP proteins are encoded in MHC class II locus. |
|
|
Post transport into ER, antigenic peptides are..
|
loaded onto newly synthesized MHC class I polypeptides. MHC class I and antigenic peptide are transported to cell surface. Stable MHC class I expression requires presence of antigenic peptide.
|
|
|
In MHC 2 Extracellular antigen is endocytosed (directly via Fc or complement receptors) and compartmentalized in cytosolic phagosomes.
|
Phagosomes fuse with lysosomes. The resulting phagolysosome degrades the microbe into antigenic peptides.
|
|
|
Newly synthesized MHC class II molecules are synthesized in the endoplasmic reticulum and transported to the phagolysosome, forming the MHC class II vesicle. The MHC class II binding cleft is occupied by
|
invariant chain (Ii) prior to antigen loading.
|
|
|
In the MHC class II vesicle, the Ii is degraded by proteolytic enzymes, leaving behind a
|
short peptide named class II-associated invariant chain peptide (CLIP).
|
|
|
_____ removes CLIP and allows antigenic peptides to be loaded in the MHC binding cleft. MHC class II and peptide are transported to cell surface.
|
HLA-DM
|
|
|
HLA-DM
|
is an intracellular protein that is involved in MHC class II antigen processing and does not present antigenic peptides nor is it a component of MHC class II.
|
|
|
MHC I Mutation, inheritence, and syndrome
|
Genes encoding for transporter associated with antigen presentation (TAP) – essential for MHCI expression.; AR; Sinopulmonary infections, granulomatous skin lesions, and necrobiosis lipoidica.
|
|
|
MHC1 Lab/Tx
|
CD8 Lymphopenia, PBMC on flow cytometry lack MHC class I.; Treat pulmonary infections like CF (aggressive toileting and chest PT)
|
|
|
MHC2 defx (Mutation/inheritence, syndrome)
|
Genes encoding for mutations in several transcription factors required for MHC class II expression: MHC2TA, RFX5, FRXAP, and FRXANK.; Diarrhea, hepatosplenomegaly, transaminitis, sclerosing cholangitis (Cryptosporidium parvum), pulmonary infections (Pneumocystis jiroveci, encapsulated bacteria, Herpesviridae, and respiratory syncytial virus [RSV]), and meningitis.
|
|
|
MHC2 defx (Lab/Tx)
|
CD4 Lymphopenia (reversed CD4:CD8) Lack of HLA DR/DP/DQ on lymphocytes, delayed-type hypersensitivity test (DTH). Hypogammaglobulinemia. Absent germinal centers from LN.; HSCT
|
|
|
Tolerance
|
is defined as unresponsiveness to an antigen. This can be to self-antigens, self-tolerance, or to foreign antigens. Self-tolerance is part of the normal function of educating the immune system not to react to itself.
|
|
|
Tolerogens
|
are antigens that induce tolerance. A foreign antigen that becomes a tolerogen is conditional. The antigen may only induce tolerance under certain conditions, like age or the amount of antigen being at a very low or high concentration.
|
|
|
Anergy
|
is a state of unresponsiveness to antigenic stimulation. The antigen is recognized by the immune cell; but weak signaling, due to a lack of costimulus, leads to anergy. Other factors include antigen type and antigen dose.
|
|
|
Central tolerance occurs is the lymph organs. T lymphocytes are educated and tolerized in
|
Thymus
|
|
|
For T-lymphocyte central tolerance, it is a T lymphocyte precursor that is exposed to a self-antigen in the thymus. The T lymphocyte is exposed to a self-antigen, which yields two fates:
|
apoptosis, which is also known as negative selection, or development into a regulatory T (Treg) cell, which will migrate to the periphery. Knowledge of T lymphocyte tolerance is limited to CD4+ T lymphocytes, including Treg cells.
|
|
|
For B-lymphocyte central tolerance, the precursor B lymphocyte is exposed to a self-antigen in the bone marrow during development. The immature B lymphocyte is exposed to self-antigen, which yields three fates:
|
apoptosis (or negative selection), receptor editing, or anergy.
|
|
|
Receptor Editing
|
reactivates recombination activation gene (RAG)-1 and RAG-2, which rearrange the Ig light chain, producing new receptor specificity.
|
|
|
Receptor editing is seen in ____ concentration of antigen.
|
high; In low antigen concentration, the B lymphocyte may become anergic to the self-antigen.
|
|
|
In both T and B lymphocytes, peripheral tolerance occurs in peripheral tissues when a mature lymphocyte encounters a self-antigen.
|
In the case of a T lymphocyte, if recognition of self-antigen occurs, the T lymphocyte may be induced to undergo apoptosis, may become anergic, or a Treg cell that confers suppression. In this situation, a B lymphocyte will either become anergic or be deleted through apoptosis.
|
|
|
The two main factors determining tolerance or negative selection are _______ and ______ to the TCR.
|
concentration and affinity; high concentration and high affinity --> negative selection
|
|
|
The thymus presents self-antigens through thymic antigen-presenting lymphocytes that process antigen in the context .
|
of HLA class I and II
|
|
|
This gene promotes expression of non-thymic tissue antigens in the thymus!
|
; AIRE, Mutation in the AIRE gene produces disorders such as autoimmune polyglandular syndrome (APS). Lymphocytes are not deleted or tolerized to endocrine-related self-antigens. The endocrine organs are attacked by autoreactive T lymphocytes and autoantibodies.
|
|
|
Peripheral T-Lymphocyte Tolerance
|
—Peripheral tolerance has the same outcomes as central tolerance: anergy, deletion, or regulation. Lack of a second signal or lack of innate costimulation (eg, micro environment) produces anergy of the peripheral T lymphocytes.
|
|
|
Anergy in these T lymphocytes is maintained by
|
TCR-signaling, ubiquitin ligases, which target proteins for degradation, and inhibitor costimulatory molecules, such as CTLA-4 and PD-1. Dendritic cells may also present self-antigen without expression of costimulator molecules
|
|
|
Once a cell is anergic,
|
costimulation will not restore activation.
|
|
|
Dendritic cells that are not activated that are or immature still present self-antigen on their surfaces. These cells in an immature state do not express receptors, thus antigen presented to T lymphocytes will
|
will not have a second signal resulting in tolerance. This presentation of antigen by the dendritic cell is ongoing, which reminds cells not to be self-reactive
|
|
|
Treg cells are mainly thymic emigrants that respond to self-antigen. Despite being self-reactive, they are allowed to escape the thymus and, instead, help to
|
maintain self-tolerance. Characteristically, they express CD25 (ie, the interleukin [IL]-2R α chain).
|
|
|
Treg cells: Their survival depends on IL-2 and transforming growth factor β (TGFβ). Tolerance or regulation is maintained by secretion of IL-10 and TGFβ.
|
IL-10 targets macrophages and dendritic cells, and TGFβ inhibits lymphocytes and macrophages.
|
|
|
Apoptosis is a key regulator of self-reacting T lymphocytes. Self-antigens repeatedly recognized by a T lymphocyte without costimulation can activate Bim,
|
proapoptotic member of the Bcl-2 protein family. Bim works through a mitochondrial pathway.
|
|
|
Cells presenting self-antigen without innate response or costimulation have other receptors on their surfaces,
|
such as Fas Ligand (FASL) (CD95L) on the T lymphocyte. FASL is upregulated on repeatedly activated T lymphocytes. FASL can interact with FAS (CD95) on the same cell or nearby cells, either deleting a self-reactive T lymphocyte or causing the death of an activated cell, thereby down-regulating the immune response. The FAS:FASL interaction signals through the caspase system.
|
|
|
Mutations in FAS or Caspase 10
|
as autoimmune lymphoproliferative syndrome (ALPS). The lymphocytes do not know when to die. They accumulate in the lymph organs. There is a lack of tolerance, producing autoimmune problems.
|
|
|
Central B-lymphocyte tolerance can include deletion or receptor editing. Receptor editing reactivates
|
RAG-1 and RAG-2 when a high affinity self-antigen is recognized by a B cell receptor (BCR). The RAG enzymes will give the BCR a new light chain.
|
|
|
___ light chains are rearranged first. If receptor editing is needed, a ___ light chain will be used.
|
kappa, lambda
|
|
|
If both recombinations recognize a self-antigen, the immature B lymphocyte
|
deleted
|
|
|
Chronic antigen recognition down-regulates CXCR5,
|
inhibiting B lymphocyte homing and interaction with T lymphocyte, which yields death.
|
|
|
Tolerance features
|
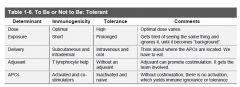
|
|
|
In central tolerance, the T lymphocyte can be deleted by negative selection of high affinity self-antigens or apoptosis; or some “self recognizers”
|
can be released into the periphery to become regulator cells to maintain tolerance.
|
|
|
B-lymphocyte central tolerance can yield deletion; but, prior to deletion, _________ may save the B lymphocyte from negative selection.
|
receptor editing
|
|
|
Peripheral tolerance can result in deletion or anergy. Anergy occurs with ____________ without a second signal and/or inflammation.
|
antigen exposure
|
|
|
A and G are purines,
|
composed of two fused rings of carbon and nitrogen.
|
|
|
T and C are pyrimidines,
|
are composed of one simple ring of carbon and nitrogen.
|
|
|
Double helix winds around ______ to form _________
|
histones; chromatin
|
|
|
X opens the chromatin to allow transcription. ____________ represses gene expression and is reduced in X
|
trasncription; histone deacetylation; COPD
|
|
|
RNA
|
Protein synthesis occurs via RNA. Composed of nucleotides. Contains the pyrimidine uracil (U), instead of T. mRNA is copied from DNA and travels to ribosome. tRNA transports amino acids to ribosome. rRNA and protein combine to make ribosomes.
|
|
|
Mutations result from changes in the nucleotide sequence of genes (Table 1-8). Germline mutations can be passed down via X
|
reproductive cells
|
|
|
Somatic mutations involve cells outside the reproductive system and generally do not get passed to
|
subsequent generation
|
|
|
Frameshift
|
Caused by the insertion or deletion of a number of nucleotides then evenly divisible by three, which causes the incorrect translation of amino acids downstream from the mutation.
|
|
|
Point
|
Type of point mutation, where a single nucleotide substitution causes the translation of a different amino acid.
|
|
|
Nonsense
|
Type of point mutation, where a single nucleotide substitution causes an early stop (or termination) codon.
|
|
|
Silent
|
Does not cause a change in amino acid sequence.
|
|
|
Neutral
|
Causes a different but similar amino acid to be translated.
|
|
|
X can be described as changes in gene function that occur without a change in the sequence of DNA. These changes occur as a result of the interaction of the environment with the genome.
|
Epigenetics
|
|
|
X AND Y likely play a crucial role in the epigenetic regulation of immune system genes.
|
DNA Methylation, Histone acetylation
|
|
|
SNP associated with increased incidence of eczema and asthma.
|
Fillagrin,
|
|
|
SNP a/w increased in asthma
|
17q12-21; ORMDL3
|
|
|
SNPs associated with both increased as well as reduced risk of development of asthma and atopy.
|
5q22-32, CD14, LPS receptor
|
|
|
SNP found to protect against nonatopic asthma.
|
3p21-22, CCR5, Chemokine Receptor
|
|
|
SNP confers increased risk for asthma, rhinitis, atopic dermatitis, and increased specific IgE.
|
XPR, TLR 7 & 8, pattern recognition receptor for viral ssRNA
|
|
|
SNP associated with increased risk of asthma, bronchial hyperresponsiveness, and skin-test responsiveness. SNP may be linked to response to montelukast.
|
5q31, IL13, Cytokine that induces IgE secretion, mucus production, and collagen synthesis.
|
|
|
Argenteum (Arg) or Arg phenotype with decreased albuterol response compared to Gly/Gly phenotype at residue 16.
|
ADRB2, B2 receptor, adrenaline/noradrenaline receptor
|
|
|
SNP confers risk of asthma and bronchial hyperresponsiveness
|
ADAM33, Type 1 membrane e pt; involved in cell/cell intxns.
|
|
|
Immunoglobulins (Igs) are glycoprotein molecules produced by B lymphocytes and plasma cells in response to an immunogen.
|
Ig is the key component of humoral immunity. The earliest cell in B-lymphocyte lineage that produces Ig is the pre-B lymphocyte. An adult human produces approximately 2-3 grams of Ig every day.
|
|
|
The Ig molecule is a polypeptide heterodimer that is composed of
|
two identical light chains and two identical heavy chains connected by disulfide bonds (Figure 1-6). Each chain consists of two or more Ig domains, which are compact, globular structures of approximately 110 amino acids containing intrachain disulfide bonds.
|
|
|
Heavy chains are designated by letters of the Greek alphabet (ie, γ, α, μ, ε, δ) for Ig classes: G, A, M, E, and D, respectively. Human IgG consists of four isotypes:
|
IgG1, IgG2, IgG3, and IgG4.
|
|
|
The constant (C) regions of IgG, IgA, and IgD consist of only X CH domains.
|
3
|
|
|
In IgM and IgE, the C regions consist of X CH domains.
|
4
|
|
|
Ig structure
|
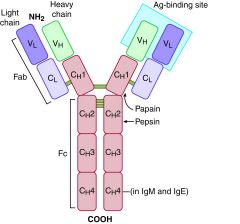
|
|
|
Light chains κ and λ are identified by their
|
C region
|
|
|
An Ig molecule has either X or Y but never one of each.
|
kk, or lambda/lambda
|
|
|
An individual B lymphocyte will produce only
|
κ or λ chains but never both.
|
|
|
The ratio of κ-bearing lymphocytes to λ-bearing lymphocytes can be used as an indication
|
clonality and is, therefore, useful in diagnosing and typing B lymphocyte lymphomas.
|
|
|
Hinge regions are X and provide Ig flexibility.
|
proline rich
|
|
|
Ig fragments are produced from enzymatic cleavage of the Ig molecule. Enzymatic digestion provides useful reagents for determining Ig function and activity. Papain cleaves Ig above the hinge (as seen in Figure 1-6) and results in X and Y fragment.
|
2 Fab (antigen binding) and one Fc (Crystallizable)
|
|
|
Pepsin cleaves Ig below the hinge at multiple sites and produces
|
F(ab′)2, which contains interchain disulfide bonds, and exhibits two antigen-binding sites..
|
|
|
F(ab) can X but not Y, F(ab')2 can _____
|
F(ab) can bind but not crosslink; and F(ab′)2 both binds and crosslinks
|
|
|
Variable regions VL and VH form the antigen-binding sites that consist of
|
complementarity determining regions (CDRs) of about ten amino acids and account for antibody diversity.
|
|
|
There are X CDRs in each V region; Y is the most variable and, typically, has the most extensive contact with the antigen.
|
3; CDR3
|
|
|
Constant regions CH and CL are located at C-terminals of the Ig molecule. Only X mediates effector functions by binding to Fc receptors or binding complement.
|
CH
|
|
|
Most variable part of the Ig
|
CDR3
|
|
|
Two forms of Ig exist that differ in the X (membrane vs secreted)
|
amino acid sequence of the C-terminal end of the CH.
|
|
|
Membrane-bound Ig (or surface Ig) is attached to the B lymphocyte surface by its transmembrane region.
|
Once Ig molecules bind to antigens and are cross-linked, they serve as B lymphocyte antigen receptors that mediate B lymphocyte activation.
|
|
|
Secreted Ig molecules lack transmembrane regions and circulate in
|
plasma, mucosal sites, and interstitial fluids. Secreted Ig can be in the form of monomers (all Ig), dimers (IgA), or pentamers (IgM).
|
|
|
Dimers or pentamers are formed by
|
tail pieces connected by disulfide bonds to joining (J) chain.
|
|
|
Affinity
|
strength of the binding between each molecule of Ig and antigen epitopes, and is indicated by Kd. A numerically lower Kd indicates higher affinity.
|
|
|
Avidity
|
It is an estimate of the overall strength of the binding between Ig and antigen. A low-affinity IgM can produce a high-avidity interaction by simultaneous binding to multiple antigen epitopes, through 10 contact sites on each IgM molecule.
|
|
|
Ig Superfamily
|
group of proteins that share similar structure to Ig by having one or more domains composed of 70-110 amino acids, most typically containing an intrachain disulfide loop. Examples of Ig superfamily members are TCR, MHC molecules, CD4, CD8, B7-1, B7-2, Fc receptors, KIR, and VCAM-
|
|
|
Ig can recognize highly diverse antigens through
|
linear and conformational determinants found in various macromolecules (ie, proteins, polysaccharides, and lipids).
|
|
|
TCRs only recognize
|
linear determinants of peptides presented by MHC molecules.
|
|
|
IgM is the first
|
produced after birth, the first to reach adult level, and the first to be synthesized following antigenic stimulation.
|
|
|
Only IgG crosses placenta. IgG level reaches a nadir around
|
four to six months after birth due to declining in passively-transferred maternal IgG.
|
|
|
IgA is produced in the highest quantity daily, and is found in higher concentrations
|
in the respiratory and GI mucosal surfaces.
|
|
|
In the gastrointestinal tract, IgA is produced by plasma cells in the lamina propria and is transported across the mucosal epithelium by
|
poly-Ig receptor (transcytosis).
|
|
|
Rheumatoid factor (RF) is an antibody against the
|
Fc portion of IgG. RF is most commonly IgM, but can also be any other isotype.
|
|
|
fixes complement most efficiently of any Ig isotype.
|
IgM
|
|
|
The shortest half-life of all IgG subclasses is IgG3.
|
IgG3
|
|
|
Ig Isotype Summary
|

|
|
|
Antigen recognition by Ig initiates a humoral immune response. Ig selectively captures antigens and microbial pathogens, including bacteria and viruses through noncovalent, reversible binding through the
|
Ig V regions.
|
|
|
Ig-mediated effector functions include:
|
neutralization of microbes or toxins, opsonization, ADCC, and immediate hypersensitivity (IgE).
|
|
|
Variability in the structures of the antigen-binding site of Ig and TCR.
|
Large antibody and TCR repertoires, potentially in excess of 109 structurally-distinct antibodies and even more TCRs.
|
|
|
Variation in inherited (germline) V, D and, J elements in Ig and TCR.
|
Inherited structural differences create different basic structural frameworks.
|
|
|
Combinatorial diversity (somatic recombination)
|
Result of different V, D, and J segment rearrangement in developing B and T lymphocytes. Moderate levels of immune receptor diversity.
|
|
|
Junctional diversity
|
Random (nontemplated) addition or removal of nucleotide sequences at junctions between V, D, and, J regions.
|
|
|
Somatic Hypermutation
|
Point mutations in V regions of Ig in rapidly dividing B lymphocytes.Selected increase (or decrease) in antibody affinity.
|
|
|
Receptor Editing
|
Changes in Ig specificity that express self-reactive antibody achieved through secondary rearrangements.
|
|
|
Somatic hypermutation leads to changes
|
V not C
|
|
|
Class switch recombination changes
|
C not V
|
|
|
Alternative splicing
|
changes Ig from transmembrane to secretory form.
|
|
|
Class Switch Recombination
|
Change in heavy chain C regions, with same V region at the gene locus.
|
|
|
Affinity maturation
|
Process of somatic hypermutation and selective survival of B lymphocytes that produce high affinity antibodies.
|
|
|
Alternative splicing
|
Splicing at different locations in the 3′ of C region exons.
Production of membrane-bound or secreted Ig forms. Splicing at different location of the C-terminal of the IgM gene. Production of IgD from the same RNA transcript with IgM. Not conventional class switch. |
|
|
Each Fc receptor functions as a receptor specific for the CH region of the Ig molecule (Table 1-12). FCRs contain domains for
|
Ig-binding and signaling components.
|
|
|
FcγRI
|
CD64; High; Macrophages, neutrophils, eosinophils; Phagocytosis
|
|
|
FcγRIIIA
|
CD16; Low; NK cells ADCC
|
|
|
FcγRIIIB
|
CD16; Low; Neutrophils Phagocytosis (poor)
|
|
|
FcεRI
|
High; Mast cells, basophils, eosinophils; Degranulation; ADCC
|
|
|
FcεRII CD23 (not 32!)
|
Low; Neutrophils, eosinophils, monocytes; Unknown
|
|
|
T lymphocyte progenitors arise in the bone morrow and travel to the thymus as
|
CD4-, CD8-, double negative, and CD3+ cells.
|
|
|
Naïve T lymphocytes recirculate through the lymph nodes looking for their unique protein antigen as displayed in the context of an HLA molecule
|
Class I for CD8 T cells and HLA Class II for CD4 T cells.
|
|
|
Naïve T lymphocytes require X to present the antigens.
|
dendritic cells
|
|
|
Effector T lymphocytes respond to
|
variety of antigen-presenting cells. (Since they have been activated, they are “ready to go”. They also remember and/or kill.)
|
|
|
The most important cytokine produced during activation is the T lymphocyte survival signal,
|
provided by IL-2 and its receptor CD 25.
|
|
|
(Common gamma chain of IL2R is shared by receptors
|
for IL2,4,7,9,15,21; mutations in common gamma chain result in XSCID)
|
|
|
Proliferation is clonal. It is stimulated by IL-2.
|
Clonal expansion preserves the specificity of the T lymphocyte for its particular antigen.
|
|
|
Effector cells react to antigens. In the CD4+ lineage, they induce differentiation of the T lymphocyte response,
|
Th1, Th2, Treg, and Th17.
|
|
|
In CD8
|
cells become cytolytic.
|
|
|
Effector cells function to eliminate antigen. With the decline in antigen stimulation,
|
there is a decline in T lymphocyte activity and achievement of homeostasis.
|
|
|
Memory cells are a subset of the clonally-expanded population.
|
These cells are long-lived and functionally quiet. They provide a rapid secondary response.
|
|
|
Lymphocyte Differentiation
|

|

