![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
98 Cards in this Set
- Front
- Back
|
Rate of reaction
|
The change in concentration of a reactant OR product per unit of time
|
|
|
Order
|
The power to which the concentration of the reactant is raised in the rate equation
|
|
|
Rate constant, k
|
The constant that links the rate of reaction with the concentrations of the reactants (raised to the powers of their orders in the rate equation)
|
|
|
Half-life
|
The time taken for the concentration of a reactant to reduce by half
|
|
|
Rate-determining step
|
The SLOWEST STEP in the reaction mechanism of a multi-step reaction
|
|
|
The half-life if a first-order reaction is ___________ of the concentration
|
independent
|
|
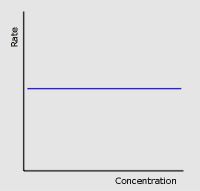
|
Zero order
|
|
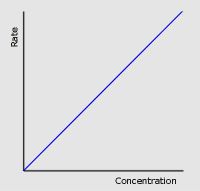
|
First Order
|
|
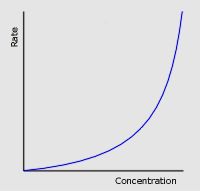
|
Second order
|
|
|
Kc =
|
PRODUCTS/REACTANTS
|
|
|
What is the effect on Kc on an increase in temperature for an exothermic reaction?
|
Kc DECREASES
|
|
|
What is the effect on Kc on an increase in temperature for an endothermic reaction?
|
Kc INCREASES
|
|
|
How is Kc affected by changes in concentration or pressure or by a catalyst?
|
It isn't affected!
|
|
|
What is an acid?
|
A species that can donate a proton
|
|
|
What is a base?
|
A species hat can accept a proton
|
|
|
What is an acid-base pair?
|
A pair of two species that transform into each other by gain or loss of a proton
|
|
|
What is the difference between a strong and weak acid?
|
A strong acid completely dissociates in solution whereas a weak acid partially dissociates.
|
|
|
What does Ka show?
|
The extent of acid dissociation
|
|
|
Weak acid, Ka =
|
[H+][A-]/[HA]
|
|
|
Large Ka =
|
Large extent of dissociation - strong acid
|
|
|
A high value of Ka means a ___ value of pKa
|
Low
|
|
|
The smaller the pKa the stronger/weaker the acid
|
Stronger
|
|
|
pKa =
|
-log(Ka)
|
|
|
pH =
10^-pH = |
-log[H+]
[H+] |
|
|
Kw = (in words)
|
Ionic product of water
|
|
|
Kw = (expression)
|
[H+][OH-]
At 25*C = 1x10^-14 |
|
|
[H+] (strong monobasic acid) =
|
[HA]
|
|
|
[H+] (weak monobasic acid) =
|
sqrt(Ka * [HA])
|
|
|
[H+] (strong base) =
|
Kw/[OH-]
|
|
|
HCOOH <--> H+ + COO-
Ka = |
[H+][COO-]/[HCOOH]
|
|
|
The stronger the acid, the _______ the value of Ka and the _______ the value of pKa
|
The stronger the acid, the GREATER the value of Ka and the SMALLER the value of pKa
|
|
|
What is a buffer solution?
|
A system that minimises pH changes on addition of small amounts of an acid or base
|
|
|
How can one make a buffer solution?
|
A weak acid and a salt of the weak acid
|
|
|
[H+] (buffer) = (2)
|
Ka * [acid]/[salt]
OR pKa + log([salt]/[acid]) |
|
|
Define: Enthalpy change of neutralisation
|
The energy change that accompanies the neutralisation of an aqueous acid by an aqueous base to form one mole of water under standard conditions.
|
|
|
Define: Lattice enthalpy
|
The enthalpy change that accompanies the formation of one mole of an ionic compound from its gaseous ions under standard conditions.
|
|
|
Explain the three trends in lattice enthalpy:
|
-ΔH
More exothermic if ionic charge increases (increased CHARGE DENSITY on the ions - stronger attraction) More exothermic if ionic radius decreases (Charge density increases if radius decreases, increased attraction) |
|
|
ΔH(solution) =
|
ΔH(hyd) - ΔH(latt)
|
|
|
Define enthalpy change of solution
|
The enthalpy change when 1 mole of a compound is completely dissolved in water under standard conditions
|
|
|
Define enthalpy change of hydration
|
The enthalpy change when 1 mole of isolated gaseous ions is dissolved in water to form 1 MOLE of aqueous ions under standard conditions
|
|
|
What is entropy?
|
A measure of the 'disorder' of a system.
|
|
|
A system becomes energetically more stable when it becomes more/less disordered?
|
more
|
|
|
Which is more entropic - solid or gas?
|
Gas
|
|
|
What happens to entropy when a solid lattice dissolves?
|
It increases
|
|
|
What happens to entropy when the number of gas particles decreases?
|
Entropy decreases
|
|
|
ΔS =
|
ΔS = S(products) - S(reactants)
|
|
|
What determines the tendency of a process to take place? (3)
|
The temperature
The entropy change The enthalpy change |
|
|
What is ΔG? What value must it take for spontaneous reactions?
|
The free energy change of a system, it must be negative for a reaction to proceed spontaneously.
|
|
|
ΔG =
|
ΔH - TΔS
|
|
|
How can endothermic reactions (positive ΔH) take place spontaneously? KEY QUESTION
|
ΔG is still negative.
ΔS is also positive - e.g. when something dissolves in water their arrangement becomes more disordered TΔS is larger than ΔH, so ΔG is negative and the reaction proceeds spontaneously. |
|
|
What is the oxidation number?
|
A measure of the number of elections that an atom uses to bond with atoms of another element.
|
|
|
What is a reducing agent?
|
A reagent that reduces (adds electrons to) another species
|
|
|
Define 'standard electrode potential of a half cell'
|
The EMF of a half cell compared with a standard hydrogen half cell, measured at 298K with solution concentrations of 1 mol dm^-3 and a gas pressure of 100 kPa
|
|
|
How does a fuel cell create a voltage?
|
It uses the energy from the reaction of a fuel with oxygen
|
|
|
Scientists in the car industry are developing fuel cell vehicles (FCVs) fuelled by:
What is the advantage of FCVs? |
Hydrogen gas and hydrogen rich fuels
Less pollution and less CO2 Greater efficiency |
|
|
How can hydrogen be stored in FCVs?
|
As a liquid under pressure
ADsorbed on the surface of a solid material ABsorbed within a solid material |
|
|
What are the limitations of hydrogen fuel cells? (3) - Get 3 points!
|
Storage and transport
Limited lifetime, high production costs Use of toxic chemicals in their production |
|
|
Hydrogen is an energy carrier, not an energy...
|
source
|
|
|
What are the limitations of a hydrogen economy? (3)
|
Public and political acceptance of hydrogen as a fuel
Handling and maintenance of hydrogen systems Initial manufacture of hydrogen (energy requiring) |
|
|
Colour: Cu2+
|
Blue
|
|
|
Colour: Cu(OH)2
|
Blue
|
|
|
Colour: Fe2+
|
Pale green
|
|
|
Colour: Fe(OH)2
|
Green
|
|
|
Colour: Fe3+
|
Yellow/orange
|
|
|
Colour: Fe(OH)3
|
Red-brown/rust
|
|
|
Colour: Co2+
|
Pink
|
|
|
Colour: Co(OH)2
|
Blue-green --> Pink
|
|
|
What is a ligand?
|
A molecule or ion that can donate a pair of electrons with the transition metal ion to form a coordinate bond.
|
|
|
What is a complex ion?
|
A transition metal ion bonded to one or more ligands by coordinate bonds.
|
|
|
What is the coordination number?
|
The total number of coordinate bonds formed between the central metal ion and any ligands
|
|
|
What is an example of a complex with a sixfold coordination number and octahedral bonding?
|
[Cu(H₂O)₆]²⁺
|
|
|
What is a bidentate ligand? What's an example of one?
|
A ligand that can donate two lone pairs of electrons to the central metal ion to form two coordinate bonds.
'en' (NH2CH2CH2NH2) |
|
|
What is the bond angle between the two groups of a cis isomer?
|
90*
|
|
|
What is the bond angle between the two groups of a trans isomer?
|
180*
|
|
|
What is the bond angle in a tetrahedral shape and when does tetrahedral bonding occur?
|
109.5*, and when all the groups are the same(?)
|
|
|
What is required for optical isomerism? (3)
|
A complex with three molecules of a bidentate ligand
A complex with two molecules of a bidentate ligand and two molecules of a monodentate ligand A complex with one hexadentate ligand ([Cu(EDTA)]2-) |
|
|
What is the use of cis-platin? How does it act?
|
It is an anti-cancer drug and its acts by binding to DNA in cancer cells, thus preventing division.
|
|
|
Colour: [Cu(H2O)6]2+
|
Pale blue
|
|
|
Colour: [Cu(NH3)4(H2O)2]2+
|
Deep blue
|
|
|
Colour: [CuCl4]2-
|
Yellow
|
|
|
Colour: [Co(H2O]6]2+
|
Pink
|
|
|
Colour: [CoCl4]2-
|
Blue
|
|
|
What's special about the bonding in [Cu(NH3)4(H2O)2]2+?
|
It's a 'distorted octohedral' shape with the Cu-H2O bonds longer than the rest.
|
|
|
[Cu(H2O)6]2+ + 4NH3 <-->
|
[Cu(NH3)4(H2O)2]2+ + 4H2O
Remember: aqueous |
|
|
[Cu(H2O)6]2+ + 4Cl- <-->
|
[CuCl4]2- + 6H2O
Remember: aqueous |
|
|
[Co(H2O)6]2+ + 4Cl- <-->
|
[CoCl4]2- + 6H2O
Remember: aqueous |
|
|
When initially adding copper(II) ions to HCl, what is the colour change?
|
Green (then yellow)
|
|
|
Why do only four Cl molecules bind to an ion instead of six?
|
They are larger and cannot fit six
|
|
|
What initially happens on addition of aqueous copper(II) ions and ammonia?
|
The solution turns blue from the precipitate of copper(II) hydroxide (Cu(OH)2). Cu reacts with OH- ions in the solution.
|
|
|
What is the importance of iron in haemoglobin?
|
It is the central ion in haemoglobin and forms four dative covalent bonds from the nitrogen atoms in a PORPHYRIN RING. It also binds to an AA on the globin molecule. The sixth ligand in the complex is OXYGEN
|
|
|
Why is CO toxic?
|
It binds to the central Fe2+ ion more strongly than oxygen and replaces it in the complex. Oxygen therefore cannot be carried around the body, causing ASPHYXIATION.
|
|
|
HbO2 + CO <-->
|
HbCO + O2
|
|
|
What is the stability constant?
|
The equilibrium constant for an equilibrium existing between a transiiton metal ion surrounded by water ligands and the complex formed when the same ion has undergone a ligand substitution.
Yeah |
|
|
What does a high value of K(stab) tell you?
|
That the equilibrium lies to the right and is stable.
|
|
|
What is important to omit in K(stab)?
|
The concentration of water
|
|
|
In the redox equation of Mn and F2, what is the ratio?
|
1 Mn to 5 Fe2+
|
|
|
What is the colour of MnO4- ions?
|
Purple
|
|
|
In the redox reaction of Mn and Fe - what colour is the end point?
|
Pink
|

