![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
73 Cards in this Set
- Front
- Back
|
Azotemia
|
containing containing
compounds, such as urea, creatinine, various compounds, such as urea, creatinine, various body waste compounds, and other nitrogen body waste compounds, and other nitrogen rich compounds in the blood related to rich compounds in the blood related to insufficient filtering of blood by the kidneys insufficient filtering of blood by the kidneys |
|
|
Uremia
|
Nitrogenous wastes + symptoms (Nausea, vomitting,disorientation, seizure, coma, death)
|
|
|
Epidemology of Acute Renal Failure (ARF)
|
Prevalence
– Healthy population - Very low ~ 0.02% – Hospitalized patient - ~7% – Chronic kidney disease - Increased risk ~ 13% – Critically ill patients - Up to 23% Mortality – 35 - 80% in hospitalized patients ARF is an independent risk factor for death |
|
|
Acute Renal Failure Outcomes
|
90% of individuals who survive recover enough renal function to live normal lives
50% of them are left with some deficits 5% do not recover renal function and require renal replacement therapy or transplantation 5% have progressive deterioration after initial recovery |
|
|
Risk factors for Acute renal failure
|
Dehydration
Pharmacologic agents Contrast media Nephrotoxic drugs Chemotherapy, NSAIDs, ACE-I, ARBs, Antimicrobials > 60 yo Male Acute infection Heart failure Respiratory failure Trauma Rhabdomyolysis Blood vessel thrombosis |
|
|
Acute Renal Failure definition
|
Decrease in glomerular filtration rate (GFR) occurring over hours to days (< 2-4 weeks)
|
|
|
Chronic Renal Failure
|
presence of proteinuria or reduced GFR (e.g. GFR < 90 ml/min) for at least 3 months
|
|
|
Commonly used criteria used to diagnose ARF
|
Absolute serum creatinine (SCr) value
Change in SCr value over time Urine output decrease |
|
|
RIFLE criteria
|
Risk — 1.5-fold increase in the serum creatinine or GFR decrease by 25 percent or urine output <0.5 mL/kg per hour for six hours
Injury — Twofold increase in the serum creatinine or GFR decrease by 50 percent or urine output <0.5 mL/kg per hour for 12 hours Failure — Threefold increase in the serum creatinine or GFR decrease by 75 percent or urine output of <0.5 mL/kg per hour for 24 hours, or anuria for 12 hours Loss — Complete loss of kidney function (eg, need for renal replacement therapy) for more than four weeks ESRD — Complete loss of kidney function (eg, need for renal replacement therapy) for more than three months |
|
|
Etiologies for Acute Renal Failure
|
Pre-Renal (Most common)
Decreased renal perfusion Intrinsic Structural damage to the kidney Postrenal Post kidney urine flow obstruction |
|
|
Normal renal hemodynamics in the afferent arteriole
|
Tone is controlled by vasodilators (PGE, PGI2)
|
|
|
Normal renal hemodynamics in the efferent arteriole
|
Tone is controlled by vasoconstrictors (Angiotensin II, Norepinephrine)
|
|
|
Functional Renal Failure
|
Decreased GFR and hydrostatic pressure at the level of the glomerulus
Decreased blood flow to the kidney decreases GFR Alteration in afferent/efferent arteriole tone No structural damage, readily reversible with increased blood flow Overlaps with causes of prerenal dysfunction Decline in GFR secondary to reduced hydrostatic pressure Afferent arteriole vasoconstriction Efferent arteriole vasodilation |
|
|
Causes of Functional Renal Failure
|
Reduced effective blood volume
CHF, Cirrhosis, Pulmonary HTN, Hypoalbuminemia (proteins increase osmotic pressure. Decreased intervascular volume - Can’t deliver enough volume to the kidneys) Renovascular disease Renal artery stenosis (- Inhibits the ability of the arteries to flex so no vasoconstriction or vasodilation) Drugs ACE-I ARB |
|
|
Hepatorenal syndrome (HRS)
|
Development of renal failure in patients with advanced liver failure (acute or chronic) in the absence of any identifiable causes of renal pathology
Type I Rapidly progressive SCr doubling or >2.5mg/dL in less than 2 weeks Type II Slow progressive renal insufficiency |
|
|
HRS Pathogenic Mechanisms
|
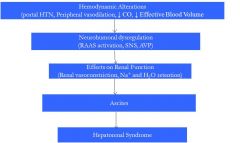
|
|
|
HRS Treatment Options
|
Vasoactive agents - )(Need to vasoconstrict the splanchnich blood supply) Vasopressin and vasopressin analogues (Improves pressure and flow to the kidney)
Octreotide + Midodrine Norepinephrine |
|
|
Treatments that don't work for HRS
|
Dopamine
Fenoldopam Diuretics |
|
|
Arginine Vasopressin
|
For the treatment of HRS:
Receptors V1a – vasoconstriction of vascular smooth muscle V2 – mediates osmoregulation and water retention V3 – Affect corticotropin secretion Splanchnic vasculature has large number of V1 receptors V1 effects increase effective arterial blood volume and suppress activation of the RAAS and SNS, reversing vasoconstriction and increase renal perfusion |
|
|
HRS Treatment Options
|
Vasoactive agents - )(Need to vasoconstrict the splanchnich blood supply) Vasopressin and vasopressin analogues (Improves pressure and flow to the kidney)
Octreotide + Midodrine Norepinephrine |
|
|
Treatments that don't work for HRS
|
Dopamine
Fenoldopam Diuretics |
|
|
Arginine Vasopressin
|
For the treatment of HRS:
Receptors V1a – vasoconstriction of vascular smooth muscle V2 – mediates osmoregulation and water retention V3 – Affect corticotropin secretion Splanchnic vasculature has large number of V1 receptors V1 effects increase effective arterial blood volume and suppress activation of the RAAS and SNS, reversing vasoconstriction and increase renal perfusion |
|
|
Vassopressin
|
Retrospective evaluation of 43 patients
Response Complete - decrease in SCr to ≤ 1.5 mg/dL Partial - 50% decrease in SCr to a value >1.5 mg/dl |
|
|
Vassopressin
|

Retrospective evaluation of 43 patients
Response Complete - decrease in SCr to ≤ 1.5 mg/dL Partial - 50% decrease in SCr to a value >1.5 mg/dl |
|
|
Vassopressin
|

Retrospective evaluation of 43 patients
Response Complete - decrease in SCr to ≤ 1.5 mg/dL Partial - 50% decrease in SCr to a value >1.5 mg/dl |
|
|
Drug-Induced Functional ARF
|
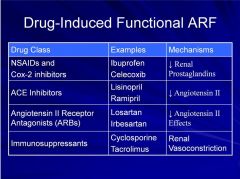
NSAIDS: Effect afferent arteriole tone
The rest Effect Efferent arteriole tone |
|
|
Prerenal ARF
|
Hypoperfusion of the renal parenchyma (e.g. nephrons)
Caused by Low effective blood volume Hypotension Hemmorrhage Dehydration Hypoalbuminemia Diuretic therapy Renal artery occlusion |
|
|
Prerenal Compensation
|
Activation of
Sympathetic nervous system Renin-angiotensin-aldosterone Antidiuretic hormone Which causes Thirst Increased fluid intake Sodium and water retention |
|
|
Functional/Pre-renal Failure Presentation
|
Increased Scr
Oliguria (< 500 ml urine per day) Decreased FENa (< 1%) Normal urine sediment, no RBC or WBC High BUN/SCr ratio |
|
|
Intrinsic ARF
|
Kidney damage
Renal vasculature Glomeruli Tubules Interstitium |
|
|
Renal Vascular Damage
|
Renal artery thrombosis
Renal vasculitis Hypertensive emergency Hemolytic uremic syndrome (HUS) Thrombotic thrombocytopenic purpura (TTP) |
|
|
Glomerular Damage
|
Low incidence ~ 5% of ARF
Systemic lupus erythematosus - rheumatologic diseases Poststreptococcal glomerulonephritis - bacterial toxins Antiglomerular basement membrane disease - Autoimmune |
|
|
Acute tubular necrosis (ATN)
Causes |
85% of all intrinsic ARF
The tubule of the kidney has high oxygen demand, so it is very sensitive to low oxygen states Causes Ischemia (decreased flow to the kidney for an extended period of time so not enough O2 Supplied to the tubules) Hypotension or vasoconstriction Extended pre-renal state Toxins Contrast dye, heavy metals, drugs (e.g. aminoglycosides, amphotericin, etc) Myoglobin, hemoglobin, uric acid |
|
|
Acute tubular necrosis (ATN)
Mechanism |
Tubular cells die and slough off into the tubular lumen causing increase tubular pressure and decreased GFR
Kidneys lose their ability to concentrate urine Prolonged exposure to ischemia or toxins cause tubular cells to die If ischemia/toxins removed before complete necrosis occurs 2-3 week maintenance phase followed by 2-3 week recovery phase where cells regenerate (2-4 weeks to regenerate once ATN is treated) |
|
|
Drug Induced ATN
|
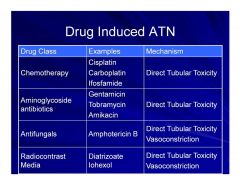
|
|
|
Rhabdomyolysis induced ATN
|
Rhabdomyolysis - statin drugs are associated with this condition
Breakdown of skeletal muscle fibers, which leads to the release of potentially nephrotoxic intracellular contents into the circulation (e.g. Myoglobinuria) Mechanisms of Rhabdomyolysis induced ARF renal vasoconstriction heme-mediated proximal tubular epithelial cell toxicity (via peroxidation) intratubular cast formation Common causes Statins, trauma, etc. Urine color heme-pigment urine |
|
|
Interstitial Damage
|
Immunologic response (monocytes, macrophages, B cells, T cells)
Widespread inflammation and edema of tissues surrounding nephrons/tubules |
|
|
Acute interstitial nephritis (Common culprits)
|
Drugs
Penicillins, ciprofloxacin, sulfonamides Infections Viral, bacteria, fungus, other |
|
|
Intrinsic Renal Failure Presentation
|
Oliguric or non-oliguric (urine > 500 ml/day)
Dilute appearing urine or discolored urine ( due to inability to concentration) Casts and cellular debris Urinary RBC or WBC Higher FENa than pre-renal (>1 because body isn’t able to reabsorb Na+ as well) Lower BUN/SCr ratio compared to pre-renal |
|
|
Postrenal ARF
|
Obstruction between the tubule and urethra
Bladder outlet obstruction Prostate hypertrophy or cancer Neurogenic bladder or anticholinergic medications Improper foley placement Ureteral Nephrolithiasis, blood clots, physical compression Renal pelvis or tubules Nephrolithiasis, oxalate, drugs Drugs – Indinavir, sulfa, acyclovir, uric acid |
|
|
Drug Induced Postrenal ARF
|
Acyclovir
Topiramate Methotrexate Indinavir Trimethoprim/Sulfamethoxazole Anticholinergic medications Cocaine |
|
|
Post-renal Presentation
|
Increased SCr
Urine output will depend on extent of obstruction Urine crystals – can see precipitates Cellular debris – not usually casts unless long term Variable FENa and BUN/SCr ratio |
|
|
Patient Assessment
|
Past medical history
Patient weight, edema Urine labs Radiographic tests (e.g. ultrasound, CT) Current medications Recent procedures |
|
|
Acute Renal Failure Assessment Summary
|
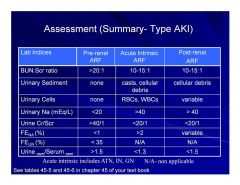
|
|
|
SCr values vs. GFR in ARF
|
SCr values lag behind what is actually going on in the kidney
A patient suffers a pre-renal ARF due to trauma or infection GFR plunges but SCr slowly builds up During recovery GFR improves but the SCr underestimates improvement due to lag in removal |
|
|
Renal Function Assessment in ARF
|
Cockcroft-Gault and MDRD
Overestimate renal function when worsening Underestimate renal function when improving Urine output over last 4 hours Abrupt decline or increase suggests change in renal function Does not evaluate quality of urine produced Solute content, waste, electrolytes, acids/bases |
|
|
Goals/approach to ARF
|
1. Prevent ARF if possible
2. Minimize further renal damage 3. Provide supportive measures until kidney function returns |
|
|
Prevention of ARF
|
Specifically targeted patients
Chronic kidney disease Elderly Comorbid conditions Recommendations Daily fluid intake (~ 2 L/day) to avoid dehydration Identify medications that put patients at risk Chemotherapy, Contrast dye, aminoglycosides, etc. |
|
|
Treatment of ARF
|
Goals of therapy
Minimize insult to kidney Shorten time to renal function recovery Restore renal function to baseline function No pharmacologic therapy to date has been shown to reverse the decline or accelerate recovery of renal function |
|
|
General Treatment Approach for ARF
|
Pre-Renal (flow issue)
Hemodynamic support Volume replacement (increase volume = increase pressure) Interstitial damage Removal of inciting agent Immunosuppressive therapy (e.g. steroids) (in) Postrenal Remove obstruction (Identify drugs that can cause crystallization in the urine) |
|
|
Supportive therapy for ARF
|
All patients need supportive therapy
Fluids for prerenal ARF (250 ml – 2 liter boluses, then maintenance rate 75-200 ml/hr) Blood pressure control (hypo and hypertension) Electrolyte balance Cardiac support if heart failure Antibiotic therapy for infection Removal of medications that decrease renal blood flow Etc. |
|
|
Electrolyte Balance Issues in ARF
|
Hypernatremia
No more than 3g/day of sodium intake Hyperkalemia potassium is 90% renally eliminated Hyperkalemia can lead to cardiac arrhythmias Reduce potassium intake Magnesium Phosphorus |
|
|
Acute Hyperkalemia
|
Serum potassium > 6 mmol/L
Life threatening Treatment Calcium chloride Sodium bicarbonate Insulin + Dextrose Albuterol nebs Furosemide Kayexalate Dialysis |
|
|
Diuretics for Fluid Management
|
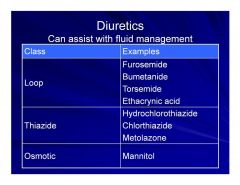
|
|
|
Diuretic resistance
|
Poor response to administered diuretics
Reduced secretion of diuretics into tubular fluid due to renal dysfunction Excessive sodium intake can override a diuretics ability to eliminate sodium Patients with ATN have a reduced number of functioning nephrons on which the diuretic can exert action Proteinuria: high amounts of protein in the tubules binds the diuretic |
|
|
Overcoming Diuretic Resistance
|
Continuous infusions (e.g. furosemide)
Combining diuretic classes Furosemide + metolazone Provides synergistic effects |
|
|
Use of Diuretics in ARF
|
Not shown to affect the course or outcome of patients with ARF
May be useful for: Volume overload in early ARF Oliguric ATN in ICU patients May combine loop with thiazide Avoid potassium sparing agents! |
|
|
Effect of Dopamine on ARF patient outcomes
|
Increases urine output, but does not decrease mortality or the need for RRT
|
|
|
Artificial kidney - dialysis
|
Used in patients who cannot be managed by supportive therapy alone
Useful for managing Uremia Metabolic acidosis Hyperkalemia and other electrolyte imbalances Excess fluid retention Accumulation of renally cleared medications |
|
|
Indications for Renal Replacement Therapy (RRT) in ARF
|
A – Acid-base abnormalities
E – Electrolyte imbalance I – Intoxications O – Fluid Overload U - Uremia SCr value - SCr does not dictate when to give dialysis Urinary Output - also does not dictate when to give dialysis |
|
|
Renal Replacement Therapy for ARF
|
Intermittent Hemodialysis
~ 4 hour treatments (4 hours that day then more the next day) Continuous Renal Replacement Therapy Continuous (e.g. 24 hour) treatment (24 hour a day dialysis can be safer, gentler, and continuously monitored) |
|
|
Comparative recovery from the different types of ARF
|
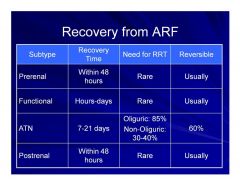
|
|
|
Contrast Induced Nephropathy (CIN)
|
Contrast agents used in patients with decreased renal function can cause ARF
Accounts for ~12% of hospital acquired ARF |
|
|
IV Contrast Agent use
|
Radiography
Interventional cardiology CT scans Etc. |
|
|
Mechanisms for CIN
|
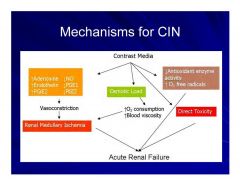
|
|
|
When comparing contrast agents
|
The lower the osmolarity, the less toxic the agent and the less ionized the better.
|
|
|
Selected Risk Factors for CIN
|
Pre-existing renal dysfunction
Diabetic renal disease Dehydration Heart failure EF < 40% Concurrent nephrotoxins High volume of contrast Age Hyper or hypotension Anemia |
|
|
Use of Fluids in Prevention of CIN
|
Contrast toxicity can occur within minutes, so interventions need to start prior to contrast administration
Fluids Dilute contrast media Prevent renal vasoconstriction Help avoid tubular obstruction Fluid choice Encourage oral fluid intake 0.9% NaCl is superior to D5W or 0.45% NaCl Example: 0.9% NaCl 1 ml/kg/hr 12 hrs before and after contrast or 0.9% 500-1000 ml bolus over 1-2 hrs prior to contrast This is the best, most important thing that can be done. |
|
|
Use N-Acetylcysteine (NAC) in the prevention of CIN
|
N-Acetylcysteine (NAC)
Antioxidant Free radical scavenger Dose 600-1200 mg q12h starting 24 hrs before contrast and continue 24hrs post contrast (4 doses) IV and PO dosing protocols with varying dosing schedules Must also give IV hydration! This is a good option. |
|
|
Use of Sodium bicarbonate in the prevention of CIN
|
Sodium bicarbonate
hypothesis that contrast injury is from free radicals generated within the acid environment of the renal medulla By increasing medullary pH, bicarbonate might protect from oxidant injury by slowing Haber-Weiss radical production (Fe3+ + O2- → Fe2+ + O2; Fe2+ + H2O2 → Fe3+ + OH + OH–) bicarbonate scavenges peroxynitrite and other reactive species generated from nitric oxide Sodium bicarbonate dose 3 ml/kg/hr x 1 hour before contrast administration followed by an infusion of 1 ml/kg/hr x 6 hours postcontrast Bicarb can slow down free radical generation and provides fluid hydration to the patient |
|
|
Use of Fenoldopam in the prevention of CIN
|
This is NOT a currently recommended therapy for prevention of CIN due to mixed study results.
Fenaldopam is a DA1 Agonist which should increase renal blood flow, increase GFR, increase natiuresis, increase diuresis and inhibits Na/K exchange |
|
|
What is the most beneficial combination therapy for the prevention of CIN?
|
Bicarbonate plus NAC
|
|
|
Glycemic Control in the prevention of CIN
|
Maintaining blood glucose 80-110 mg/dL vs. 180-200 mg/dL in surgical ICU patients
|

