![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
Rate of reaction
|
-The change in the concentration of reactants or products per units time
|
|
|
Simple collision theory: Before a reaction can occur, what two things must happen?
|
-Collide with the correct activation energy -Collide in the correct orientation |
|
|
What is Activation energy?
|
-Minimum energy required for a reaction to take place
|
|
|
Why do most collisions not results in a reaction?
|
-Most particles do not have energy that is equal to or more than the Activation energy required for the reaction to take place.
|
|
|
Factors affecting the rate of reaction
|
-Pressure -Temperature -Concentration -Surface area -Catalyst |
|
|
If Concentration increases, why does the rate of reaction increase?
|
-The number of particles pre unit volume increases -So the number of successful collisions per second increases |
|
|
If Pressure increases, why does the rate of reaction increase?
|
-The number of particles per unit volume increases -So the number of successful collisions per second increases |
|
|
If Surface area increases, why does the rate of reaction increase?
|
-If the sample of a solid is crushed into smaller pieces, the surface area of the solid will increase. The solid particles are exposed to the other reactants and there should be more collisions per second. |
|
|
If Temperature increases, why does the rate of reaction increase?
|
-At higher temperatures particles have more energy and collide more often. -The average energy of the particles increases -So the particles are moving faster -More particles have a collisions energy greater than the activation energy. -So the number of successful collision per second increases |
|
|
What is a catalyst?
|
-A substance which changes the rate of a chemical reaction without being chemically altered at the end of a reaction
|
|
|
How do catalyst work?
|
-Provides an alternative reaction pathway which has a lower activation energy than the un-catalysed reaction.
|
|
|
Maxwell-Boltzmann Distribution Curves
|
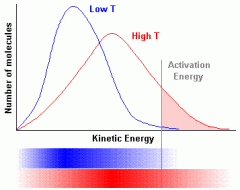
-Distribution of energies amongst the particles -Concentration raises the second curve ^^ -Catalyst increases eA |

