![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
25 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
ATP is short for |
adenosine 5' triphosphate |
|
|
|
buffer |
a solution of the acid(HA) and base (A) form of a compound that undergoes little change in pH when small quantities of strong acid/base are added |
stay in that pH |
|
|
simple sugar 3가지 |
glucose galactose fructose |
|
|
|
보류 diffusion and osmotic pressure generation 차이 |
분자의 자유 운동에 의함,한점으로부터diffusiin/ 농도 차이에 의함 아니냐?? |
|
|
|
AS AN ENGINEER,ENGINEER'S SKILLS IN |
MATHEMATICAL MODELING&UNDERSTANDING OF SCIENCE. |
즉 engineering analysis of a System |
|
|
two types of bond formed between atoms |
ionic . covalent. |
|
|
|
integumentary system |
피부계통 외피시스템 |
|
|
|
musculoskeletal system |
근골격계 |
|
|
|
renal system |
신장계 |
|
|
|
endocrine system |
호르몬을 분비하는 기관들 |
내분비계 |
|
|
intestinal system |
소화기 계통 |
|
|
|
mucus epithelial surfaces |
점액 상피 표면 |
괜찮다 계속 배우다보면 더 잘 이해 될테니까 일단은 앞으로 간다 |
|
|
resilient |
회복력 |
사람,Resilience involves the ability to recover and rebound from challenges and setbacks. |
|
|
나는 충분히 "목표지향적인가?" |

그리고 공학, 사업 |
|
|
|
allometric |
상대성장방정식,주로 allometric scaling:One common method for predicting human doses is allometric scaling. In allometric scaling, PK data from nonclinical studies in one or more animal species are used to predict human drug exposure for a range of drug doses |
ALLOMETRY: The study of size and its consequences |
|
|
백지복습 |
수술의 또한 |
|
|
|
solvent |
mostly water |
|
|
|
expenditure |
지출,경비 An expenditure represents a payment with either cash or credit to purchase goods or services. |
outlay |
|
|
thermodynamic behavior of compound |
H enthalpy+Heat of formation 이용하여 방출열
S entropy
G Gibbs free energy+possibility&favorable |
|
|
|
From standard state, Enthalpy diagram |
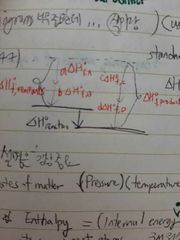
임시 |
not entropy. f for formation. |
|
|
Enthalpy = () + ()*() |
internal energy + Volume*Pressure |
internal energy 중요,V랑P는 주변이 시스템에 대해 한 일work |
|
|
its conponent atoms means |
구성원소 |
|
|
|
Enthalpy change |
overall heat kJ/mole |

첨자 못써성 델타헤이치엪 |
|
|
subscript o means that the energy is measured at |
standard condition 25degrees 1atm |
|
|
|
Table of () can be used to calculate () for other kinds of reaction |
Heat of formation enthalpy change |
|

