![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
54 Cards in this Set
- Front
- Back
|
Purpose of acute inflammation: |
Sentinel cells found microbial insult and/or tissue damage, and need reinforcement
1. Delivery of leukocytes and plasma proteins to site of injury - vasodilation - vascular permeability - emigration, activation and chemotaxis of leukocytes (leave blood and go into tissues) 2. Elimination of agent (effector processes) 3. Trigger acquired immunity 4. Initiate tissue repair 5. Systemic responses - fever, leukocytosis etc. |
|
|
5 signs of acute inflammation |
1. Rubor (redness) 2. Tumor (swelling) 3. Calor (heat) 4. Dolor (pain) 5. Functio Laesa (loss of function)
*mainly consequences of local vascular changes |
|
|
Initiators of acute inflammation: |
Infections: - bacteria - viruses - parasites - microbial toxins Trauma Physical and Chemical injury: - cold (frostbite) - burns - irradiation Tissue Necrosis Foreign body: - dirt - suture Immune Reactions: - hypersensitivities - autoimmune diseases |
|
|
What happens when sentinel cells detect microbial or parasitic products in the host tissues? |
Sentinel cells such as macrophages express PRRs so they can monitor tissues for PAMPS/DAMPS
When they find a PAMP, it leads to signaling cascades within the macrophage, which activates it
The macrophage then synthesizes and releases proinflammatory cytokines (ILs, TNF) or chemokines that act as signals for other inflammatory cells (to release even more) and blood vessels in the area
These chemokines/cytokines set up a concentration gradient as they move away from the macrophage and that helps to direct other luekocytes like neutrophils to the site of insult = chemokine-directed chemotaxis |
|
|
How are mast cells involved in acute inflammation? |
Cytokines produced by macrophages activate the mast cell to rapidly amplify proinflammatory signals
Mast cells sit near blood vessels and nerves and contain granules with histamine and serotonin (vasoactive compounds), associated with type 1 hypersensitivities (allergies)
the degranulation of mast cells (ie. release of serotonin and histamine) causes changes to local vasculature |
|
|
How are eicosanoids and cytokines different? |
Cytokines are proteins, can travel further distances
Eicosanoids (leukotrienes and prostaglandins) are oxidized derivatives of fatty acids - short half life and act locally on vasculature and endothelium |
|
|
What are the vascular changes that occur in acute inflammation? |
1. Vasodilation and local stasis of blood flow 2. Increased vascular permeability 3. Expression of cell-adhesion molecules on vascular endothelium |
|
|
What are the mediators of local vascular changes? |
1. Histamine, serotonin 2. Nitric Oxide 3. Prostaglandins and leukotrienes |
|
|
Histamine: |
Mast cell degranulation adjacent to blood cells
Induced by: - trauma/cold/heat - immune reactions (IgE) - C3a and C5a (from complement activation pathway) - Histamine releasing proteins from leukocytes - Neuropeptides (substance P) - Cytokines (IL-1 and IL-8)
Binds to H1 receptors on endothelial cells
Dilation of arterioles and increased permeability of venules |
|
|
Nitric Oxide: |
- Released from endothelial cells and macrophages - Induces relaxation of vascular smooth muscle - Also anti-inflammatory role (reduces leukocyte recruitment) - NO is microbicidal |
|
|
Prostaglandins and leukotrienes |
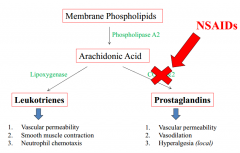
NSAIDs block COX1&2 for prostaglandin production |
|
|
What do leukotrienes increase? |
1. Vascular permeability 2.Smooth muscle contraction 3.Neutrophil Chemotaxis |
|
|
What do Prostaglandins increase? |
1.Vascular permeability 2. Vasodilation 3. Hyperalgesia (pain receptors) |
|
|
Why is vasodilation and stasis of blood flow important in acute inflammation? |
- Increased blood to area - Expansion of capillary beds - Decreased velocity of flow - Increased permeability (fluid loss)
All allow more leukocytes to travel to site of injury/infection, blood slows down enough so that the leukocytes can exit the blood into the tissues |
|
|
What do NSAIDs block? |
COX-1&2 ie. meloxicam, phenylbutazone, etc
Local effects- anti-inflammatory and analgesic Central effects - can temporarily reduce the bodys set point (anti pyretic) |
|
|
What is the action of prostaglandin E2 and what is its primary source? |
Action: Vasodilation, vascular permeability, pain
Source: Leukocytes (mast cells) |
|
|
What are some other major mediators of inflammation? |
Cytokines and chemokines Interleuken, TNF-alpha Plasma proteins Leukotrienes Eicosanoids Histamine and serotonin |
|
|
What is vasodilation? |
Vascular smooth muscle contraction
-increased blood to area, -expansion of capillary beds, -Decreased velocity of flow, -Increased permeability (fluid loss) |
|
|
What is vasodilatation mediated by? |
Histamine Nitric Oxide Prostaglandins |
|
|
Increased vascular permeability physically is?
|
-Gaps between endothelial cells due to endothelial contraction or trauma
|
|
|
Increased vascular permeability causes what? |
-transport of plasma through endothelial cells (transcytosis) -High protein fluid accumulates in tissue = edema -Edema activates endothelia cells to make inflammatory mediators |
|
|
What is increase vascular permeability mediated by? |
Histamine Leukotrienes Protaglandins |
|
|
Why is there a decrease blood velocity? |
-Vasodilation (expansion of capillary beds) -Increased permeability (fluid loss) |
|
|
What can cause vascular changes? |
-Endothelial contraction -Increased Transcytosis -Direct Injury -Leukocyte dependent injury |
|
|
What are the four phases of leukocyte extravasation? (blood to tissue) |
1. Leukocyte margination (tethering) and rolling 2. Firm endothelial adhesion (pavementing) 3. Diapedesis (transmigration) 4. Migration in interstitial tissues - chemotactic gradient (chemotaxis) |
|
|
Migration and rolling... -Can be normal in blood vessels but do not adhere to epithelium -Vasodilation and increased _________, _________, and slows blood flow |
-Can be normal in blood vessels but do not adhere to epithelium -Vasodilation and increased vascular permeability, hemoconcentrates and slows blood flow |
|
|
What causes the activation of endothelial cells to be able to tether leukocytes cis 'selections'? |
TNF-alpha, IL-1, and histamine |
|
|
Migration and rolling causes leukocytes to transiently adhere to the endothelium (rolling). This allows for what?
What does the presence of chemokines activate? |
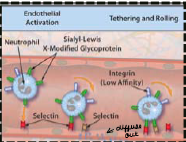
-Close association between endothelium and leukocytes
-High affinity integrins on the leukocyte that allow them to adhere |
|
|
What is a disease that lacks the ability to for leukocytes to adhere? |
Bovine leukocyte adhesion deficiency -Holstein cattle (autosomal recessive) - gene B2-integrin -Impaired expression of LFA-1 on neutrophils thats required for firm adhesion -Rolling neutrophils cannot adhere = unable to extravasate
**re-occuring bacterial infections and premature death |
|
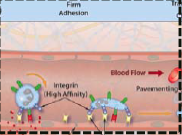
Describe firm adhesion (pavementing) ...
|
-chemokines and rolling leukocytes causes change from low to high affinity integrin surface expression -firm integrin-mediated binding of leukocytes to endothelium at infection site =leukocytes spread out along endothelial surfaces (pavementing) |
|

Describe Diapedesis…. |
-predominantly in venules -basement membrane is compromised by leukocytes secretion of collagenases (digest membrane) |
|

Describe Chemotaxis... |
-moves along chmoattractant gradient -Extended cytoplasmic tag, then pulls trailing edge (polymerization of actin then pseudopodia formation) |
|
|
What are the two types of chemoattractant gradients? |
Exogenous: N-formyl-methionine and bacterial lipids
Endogenous: C3a C5a (complement), leukotrienes, IL-8 |
|
|
Main cell in pus? |
Neutrophils |
|
|
Main cell with parasites? Mechanism? |
Eosinophils (cells with red cytoplasmic granules)
Mechanism: combination of chemokines and cytokines, type adhesion molecules on endothelia |
|
|
Appearance of leukocytes during inflammation |
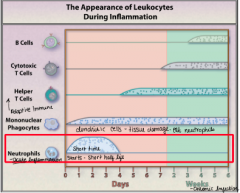
|
|
|
What are some systemic effects of acute inflammation? |
-loss of appetite -slow wave sleep -lethargy -cachexia (muscular wasting) -Hemodynamic changes (shock): decreased vascular resistance (vasodilation) and increased heart rate -Leukocytosis (neutrophilia) -metabolic acidosis (low blood pH) -Acute phase protein alterations -Fever (pyrexia) |
|
|
What is the purpose of the systemic effects? |
To conserve energy and mobilize reserves |
|
|
What are the 3 organs that produce acute inflammation? |
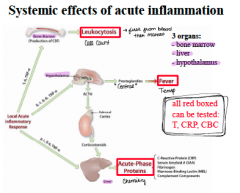
-Bone marrow = leukocytosis -Liver = acute phase proteins -Hypothalamus = Fever (can have affect on liver) |
|
|
Leukocytosis… |
-Initial increase in circulating neutrophils (IL-1 and TNF increase release of stored neutrophils in bone marrow) -Prolonged infection induces production of colony stimulation factors |
|
|
Prolonged infection induces production of colony stimulation factors which do what? |
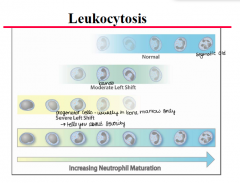
Release of immature (band) neutrophils (left shift) -Neutrophilia with left shift is the most common in change in acute inflammation |
|
|
Acute phase proteins (APP) are synthesized where? |
In the liver in response to cytokines in fist 24 - 48 hours |
|
|
What are the actions of acute phase proteins? |
-homeostasis of tissue -reduces tissue damage -aids in propagating inflammatory reaction -Regulation of immune response -Protection against infection -Aids in tissue repair |
|
|
Examples of acute phase proteins |
C-Reactive Protein (CRP): Dog and pig, not cats
Serum Amyloid A: Dogs, cats, pigs, and cattle
Haptoglobin:Pigs, cattle, sheep -moderate in cats and dogs |
|
|
Acute phase proteins - Positive APP and negative APP examples |
Positive -C-Reactive Protein -Serum Amyloid A (SAA) -Haptoglobin -Alpha-1-acid glycoprotein (AGP) -Ceruloplasmin -Fibrinogen
Negative -Albumin -Transferin |
|
|
Fever: Pyrogens stimulate prostaglandin systhesis in vascular and perivascular cells of the ______
Pyrogens include:??? |
Hypothalamus
Pyrogens = LPS (exogenous) -IL-1 and TNF (endogenous) |
|
|
Function of fever…. |
-Induction of heat shock proteins (enhance leukocyte response) -Increase expression of cytokines -Enhance phagocytosis -Increased t cell proliferation -Inhibition of growth of some microbes
|
|
|
Thermoregulation of body - overview |
Cutaneous and deep receptors or monoamines => stimulate anterior hypothalamic area of brain => Heat gain or Heat loss |
|
|
Heat gain |
Heat production - Catecholamines, thyroxine, or shivering
Heat loss - vasoconstriction, piloerection, postural changes, behavioural changes
|
|
|
Heat loss |
Panting Vasodilation Postural changes Perspiration Behavioural changes |
|
|
What does fever feel like? |
1. Body's thermostat is set to a higher temperature (body is lower than thermostat): patient is cold -> shivers
2. Temperature reaches set point (not hot or cold): high temperature measured -> fever
3. Body brings temperature back down and thermostat is at a lower temp (body is higher than thermostat): patient is hot 'breaking the fever' -> sweating **infection under control |
|
|
Signs of acute inflammation... |
1. redness: increased vasodilation due to vasoactive mediators dilating vessels 2. Heat: vasodilation and blood flow increase 3. Swelling: leakiness of blood vessels causes plasma to leak from blood to produce edema 4. Pain: prostaglandins, neuropeptides, cytokines 5. Loss of function |
|
|
Test used for mastitis? |
California mastitis test (agglutination of DNA)
OR automated cell counting |
|
|
Mastitis... |
Usually bacterial infection -PAMPs, sentinel cells, acute inflammation -Mediators, vascular changes, leukocyte recruitment -systemic effects
Blood: left shift, haptoglobin, SAA |

