![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
How do various matter change state?
|
Solid (melts) to --> liquid (boils) to --> gas (condenses) to --> liquid (solidifies) to --> solid
|
|
|
What theory explains the three states of matter?
|
Kinetic theory!
|
|
|
What are the three states of matter?
|
Solid (e.g. ice), liquid (e.g. water), gas (e.g. water vapour)
Particles are the same, just the arrangement different |
|
|
What's the arrangement of particles in solids?
|
strong forces of attraction hold particles close together in fixed arrangement.
Not much energy so vibrate about fixed positions. |
|
|
What arrangement of particles in liquids?
|
Weaker forces of attraction between particles.
Particles are close together but can move past each other to form irregular arrangements. More energy than particles of solid. random directions and low speeds. |
|
|
What arrangement for gases?
|
Almost no forces of attraction between particles.
More energy than solid/ liquid. free to move. high speed random directions. |
|
|
What happens when you heat a liquid?
|
heat energy makes particles move faster.
particles gain enough energy to overcome attraction to each other and gas forms (boiling) |
|
|
What happens when you heat a solid?
|
Heat energy makes particles vibrate faster until forces between them are overcome and particles start to move around (melting)
|
|
|
What is the melting point?
|
The temp at which it turns from solid to liquid.
boiling point is temp at which liquid becomes gas. |
|
|
What is evaporation?
|
Particles escape from a liquid and become gas
Can evaporate at temps much lower than boiling point. |
|
|
How does evaporation happen?
|
Particles near surface can escape and become gas if:
1. particles are travelling in right direction to escape liquid 2. particles travelling fast enough (kinetic energy) to overcome attractive forces of other particles in liquid. |
|
|
How does evaporation cool you?
|
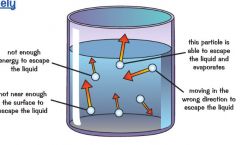
1. when particles leave liquid then average speed and kinetic energy decreases.
2. decrease in average particle energy means the temp of remaining liquid falls (cools). |

