![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
what is the structure of an erythrocyte?
|
-7.5 x 2.0 micrometres
-biconcave dsic -flexible cytoskeleton andouter lipid bilyer with hydrophobic skin -no nucleus of organelles - allow for shape and maximum concentration of Hb |
|
|
Hb gas exchange picture
|
|
|
|
what is Le Chatelier's principle?
what is it's significance in gas exchange in Hb |
if a chemical system at equilibruim experiences a change in concentration, temperature, volume of partial pressure, then the equilibruim shifts to counteract the change and a new equilibrium in established
if Hb is in a area of high CO2 (at respiring tissues) the equiibrium will be driven to release O2, and visa versa |
|
|
some functions of Hb
|
-picks up, carries, and delivers O2 and CO2
-acid-base buffer |
|
|
describe the structure of Hb
|
GLOBIN
-- 4 globular proteins (2 alpha and 2 beta) each with hydrophobic pocket, each containing HAEM --PROPHORIN ring with Fe++ in the middle (some have Fe+++ but do not carry O2) --gives blood red colour ----oxygenated - bright red ----deoxygenated - dark red |
|
|
changes to structure of Hb during oxygenation
|
- binding of O2 rearranges electrons so iron becomes smaller
- this allows it to move into the plane of the PORPHYRIN ring - this has an ALLOSTERIC effect, substantially changing the structure of the whole Hb molecule, from TENSE to RELAXED state - increasing its affinity for O2 |
|
|
wee diagram showing change brought on by O2 binding to Hb
|

|
|
|
features of myoglobin
|
-found in muscle
-only one chain, with one haem group -'soaks up' O2 -much greater affinity for O2 then Hb |
|
|
O2 dissociation curve for Hb and Mb
|

|
|
|
affects of CO2 levels on Hb affinity for O2
|
when CO2 is high, Hb's affinity for O2 is reduced
when CO2 is low, Hb's affinity for O2 is increased |
|
|
what is BPG and how does it affect Hb's affinity for O2?
|
2-3 bisphosphoglycerate
formed in RBC as a byproduct of GLYCOLYSIS decreases Hb's affinity for O2, effectively changing it to it's TENSE form (thereby supporting O2 delivery) |
|
|
what are the effects of increased acidity on Hb's affinity for O2?
|
increased acidity (ie lower pH) reduced Hb's affinity for O2 (supporting O2 delivery)
|
|
|
how does temperatue affect Hb's affinity for O2?
|
higher temperature reduces Hb's affinity for O2
|
|
|
what happens with Hb and O2 and all that junk in anaemia?
|
- the O2 carrying capacity of the blood is reduced
- BUT there is some sort of magical adaptation whereby Hd in anaemia delvers more O2 than would be expected (may have something to do with 2-3 BPG) |
|
|
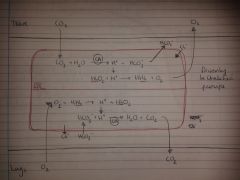
4 ways CO2 is transported in blood
|
-in blood plasma
-in Hb - carbaminohaemoglobin (not on haem site) -in RBC as HCO3- (most of it is in this way) -in plasma as HCO3- |
|
|
tell me about carbon monoxide poisoning please
|
-CO has about 200x the affinity of O2 to Hb (0.05% in air will bind 50% of Hb)
-CO binds irreversibly to haem groups, making bright red carboxyhaemoglobin -increases a haem molecule's affinity for O2, so O2 stays in the blood instead of being delivered to the tissues -ain't no-one needs that |

