![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
|
Electron shells |
Shells are energy levels
Surround the nucleus The energy increases as the shell number increases The shell number or energy level number is called the principal quantum number |
|
|
Atomic orbitals |
Shells are made up of atomic orbitals
An orbital is a region around the nucleus that can hold up to two electrons with opposite spins Models show orbital as a space where an electron is likely to be An orbital can hold either one or two electrons but no more There are different types of orbital: s-, p-, d- and f- Each types of orbital has a different shape |
|
|
S-orbitals |
Electron cloud in the shape of a sphere
Can hold one or two electrons Each shell from n=1 contains one s-orbital The greater the shell number n, the greater the radius of the s-orbital |
|
|
S-orbital shape |
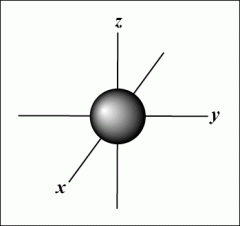
|
|
|
P-orbitals |
Electron cloud is in the shape of a dumb-bell
Can hold one or two electrons 3 separate p-orbitals at right angles to each other Each shell from n=2 contains 3 p-orbitals The greater the shell number n, the further the p-orbital is from the nucleus |
|
|
P-orbital shape |
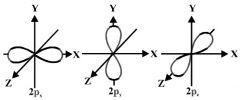
|
|
|
D- and f- orbitals |
D- and f- orbitals are more complex
Each shell from n=3 contains 5 d-orbitals Each shell from n=4 contains 7 f-orbitals |
|
|
Sub-shells |
A new type of orbital is added for each additional shell Within a shell, orbitals of the same type are grouped together as sub-shells shell sub-shells no. of electrons 1 1s2 2 2 2s2 2p6 8 3 3s2 3p6 3d10 18 4 4s2 4p6 4d10 4f14 32 |
|
|
No. of electrons in each sub-shell |
Sub-shell no. of sub-shells max. no. of electrons s 1 2 p 3 6 d 5 10 f 7 14 |
|
|
Filling of orbitals - order of increasing energy |
The sub-shells that make up the shells have slightly different energy levels
Within each shell, the new type of sub-shell added has a higher energy Such as n=4 shells fills as 4s, 4p, 4d, 4f Exception where 3d sub-shell is at a higher energy level than the 4s sub-shell So 3d fills before 4s so order is 3p, 4s, 3d When making an ion remove from 4s first then 3p |
|
|
Filling of orbitals order picture |
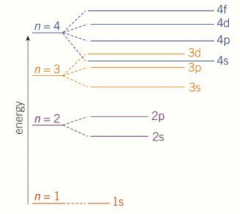
|
|
|
Filling of orbitals - opposite spins |
Each orbital can hold up to two electrons Electrons are negatively charged and repel each other Electrons have a property called spin - either up or down Electrons are shown in boxes with the arrows up or down to show spin Electrons in an orbital must have opposite spins to counteract repulsion between the negative charges |
|
|
Electron boxes and pairing |
Within an a sub-shell, the orbitals have the same energy One electron occupies each orbital before pairing starts This prevents any repulsion between paired electrons until no more orbitals available at the same energy level |
|
|
Electron boxes picture |

|
|
|
Electronic configuration |
Shows how sub-shells are occupied by electrons Krypton 36 electrons configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 |
|
|
Shorthand electronic configurations |
Electron configurations expressed more simply in terms of the previous noble gas plus the outer electron sub-shells Na = 1s2 2s2 2p6 3s1 = [Ne]3s1 K = 1s2 2s2 2p6 3s2 3p64 s1 = [Ar]4s1 |
|
|
Electronic configuration of ions |
Positive ions (cations) are formed when atoms lose electrons Negative ions (anions) are formed when atoms gain electrons |
|
|
Blocks of the periodic table |
The periodic table is divided into blocks corresponding to their highest energy sub-shell S-block - highest energy electrons in the s-sub-shell - left block of 2 groups P-block - highest energy electrons in the p-sub-shell - right block of 6 groups D-block - highest energy electrons in the d-sub-shell - centre block of 10 groups |
|
|
Ions of s- and p-block elements |
When forming ions, the highest energy sub-shells lose or gain electrons O = 1s2 2s2 2p6 3s2 3p4 O 2- = 1s2 2s2 2p6 3s2 3p6 |
|
|
Ions of d-block elements |
The 4s shell is filled before the 3d Once filled the energy level of 3d falls below 4s so the 4s empties before the 3d shell 4s is first in and first out |
|
|
Ionic bonding |
Ionic binding is the electrostatic attraction between positive and negative ions
It holds together cations and anions in ionic compounds Common cations - metal ions, ammonium ions Common anions - non-metal ions, polyatomic ions |
|
|
Ionic dot-and-cross diagrams |
Simplest contain a metal and a non-metal
Outer electrons from a metal atom are transferred to the outer shell of a non-metal atom Positive and negative ions are formed The ions formed have outer shells with the same electron configuration as the nearest noble gas |
|
|
Ionic dot-and-cross picture |
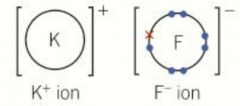
|
|
|
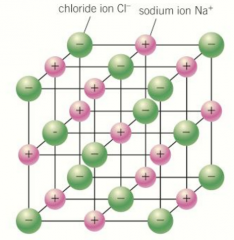
Structure of ionic compounds |
Each ion attracts oppositely charged ions in all directions This results in a giant ionic lattice structure containing billions of ions In NaCl: Each Na+ ion surrounded by 6 Cl- ions Each Cl- ion surrounded by 6 Na+ ions Each ion is surrounded by oppositely charged ions to form a giant lattice Physical properties explained from the giant ionic lattice and ionic bonding |
|
|
Structure of ionic compounds diagram |
|
|
|
Ionic compound - melting and boiling points |
Almost all ionic compounds are solids at room temperature At room temperature insufficient energy to overcome the strong electrostatic forces of attraction between oppositely charged ions in a giant ionic lattice High temperatures are needed to provide the large quantity of energy to overcome the strong electrostatic attraction between ions Therefore the compounds must have a high melting and boiling point Melting points are higher which contain ions with greater ionic charges as more attraction between ions such as Ca2+ stronger than Na+ |
|
|
Ionic compound - solubility |
Many ionic compounds dissolve in polar solvents such as water Polar water molecules break down the lattice and surround each ion in solution In a compound with ions with large charges , the ionic attraction may be too strong for water to break down the lattice The compound then will not be very soluble The higher the charge on the ion the less soluble it is |
|
|
Ionic compounds - electrical conductivity |
In the solid state, an ionic compound does not conduct electricity because the ions are in a fixed position in the giant ionic lattice so there are no mobile charge carriers Ionic compounds dissolved in water or melted conduct electricity because the solid ionic lattice breaks down and the ions are now free to be mobile charge carriers |
|
|
Covalent bonding |
Covalent bonding is the electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms Covalent bonding occurs between atoms in: Non-metallic elements for example H2 and O2 Compounds of non-metallic elements such as H2O and CO2 Polyatomic ion such as NH4+ They have a shared pair of electrons between the two atoms Can be a single molecule, giant covalent structure or a polyatomic ion |
|
|
Orbital overlap |
A covalent bond is the overlap of atomic orbitals, each containing one electron, to give a shared pair of electrons The shared pair of electrons is attracted to the nuclei of both bonding atoms The bonded atoms often have outer shells with the same electron structure as the nearest noble gas Covalent bond different from ionic as it is localised, acting solely between the shared pair of electrons and the nuclei of the bonded atoms It gives small units called molecules |
|
|
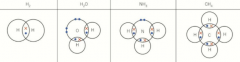
Dot-and-cross diagrams |
Lone pairs are a pair of electrons that aren’t bonded Bonded pairs are in a covalent bond |
|
|
Covalent dot-and-cross |
|
|
|
Number of covalent bonds C N O H |
Carbon - 4 covalent bonds Nitrogen - 3 covalent bonds Oxygen - 2 covalent bonds Hydrogen - 1 covalent bond |
|
|
Boron |
Period 2 and electronic configuration of 1s22s22p1 so only 3 outer shell electrons to be paired Boron forms covalent compounds such as BF3 So sometimes has fewer than full electron shells BF3 has 6 |
|
|
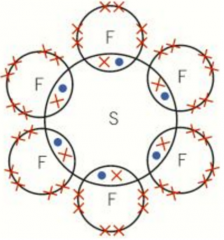
Phosphorus, sulfur and chlorine |
All in period 3 Can undergo expansion of the octet and possible only from the n=3 shell in the d-sub-shell expansion The n=3 outer shell can hold 18 electrons so more electrons for bonding Such as sulfur forms SF2, SF4 and SF6SF6 has 12 electrons in its outer shell |
|
|
SF6 dot-and-cross diagram |
|
|
|
Multiple covalent bonds |
When two atoms share more than one pair of electrons Double covalent bonds Electrostatic attraction between two shared pairs of electrons and the nuclei of the bonded atoms for nearest noble gas structure Such as O=O and O=C=O Triple covalent bonds Electrostatic attraction between three shared pairs of electrons and the nuclei of the bonding atoms for nearest noble gas structure Such as N=_N and H-C =_N |
|
|
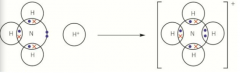
Dative covalent bonds |
A dative covalent (coordinate bond) is a covalent bond in which the shared pair of electrons has been supplied by one of the bonding atoms only The shared pair was originally a lone pair of electrons An ammonia molecule donates its lone pair of electrons to a H+ ion In displayed formula it is showed with an arrow head |
|
|
Dative covalent bond ammonia |
|
|
|
Average bond enthalpy |
Measures covalent bond strength The larger the average bond enthalpy, the stronger the covalent bond |

