![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
80 Cards in this Set
- Front
- Back
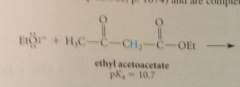
B-keto esters like malonic esters
|
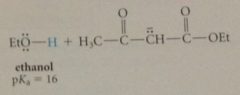
more acidic than ordinary esters, completely ionized by alkoxide bases
|
|
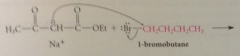
enolate ions derived from b-keto esters like malonate ester derivatives
|
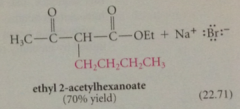
can be alkylated by primary / unbranched secondary alkyl halides or sulfonate esters
|
|
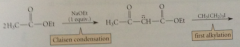
Dialkylation of B-keto esters also possible
|
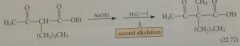
|
|
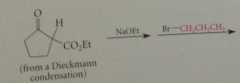
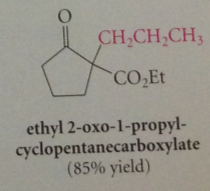
Alkylation of Dieckmann cond prod is same type of rxn
|
|
|
|
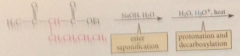
Like esters of sub malonic acids, alkylated derivatives of ethyl acetoacetate can be
|
hydrolyzed & decarboxylated to give ketones
|
|
|
Ester saponification & protonation gives
|
substituted B-keto acid
|
|
|
B-keto acids spont decarboxylate @
|
room temp
|
|
alkylation of ethyl acetoacetate followed by saponification, protonation, decarboxylation to give a ketone
|
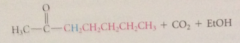
acetoacetic ester synth
|
|
|
whether a target ketone can be prepared by acetoacetic ester synth can be determined by
|
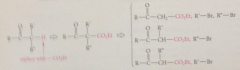
mentally reversing synth
|
|
|
replace a-H of target ketone w
|
-CO2Et group- unveils B-keto ester required for synth
|
|
|
B-keto ester can be prepared by Claisen cond or
|
from other B-keto esters by alkylation or dialkylation w appropriate alkyl halides
|
|
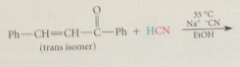
conj arrangement of C=C & C=O bonds endows a,b unsat carbonyl groups w unique reactivity
|
|
|
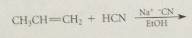
|
|
|
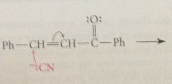
nuc add to db in a,b-unsat carbonyl cmpd occurs bc
|
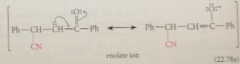
gives resonance-stabilized enolate ion intermediate
|
|
|
enolate ion can be protonated on O or C
|
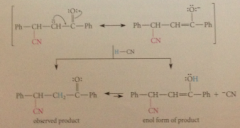
either way, carbonyl group eventually regen bc enols spont form carbonyl cmpds - overall result of rxn is net add to db
|
|
nuc add to C-C db of a,b-unsat aldehydes, ketones, esters & nitriles
|
general reaction can be observed w variety nucs
|
|
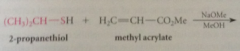
|

|
|
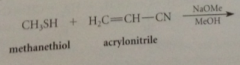
|
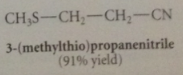
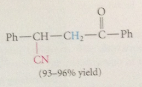
addition of cyanide forms new C-C bond, nitrile then converted into CA group by hydrolysis
|
|
|
cyanoethylation
|
add of nuc to acrylonitrile
|
|
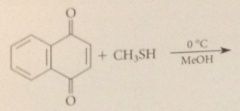
|
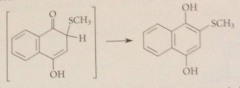
driven to completion by enolization of ketone in brackets to phenol, which is aromatic
|
|
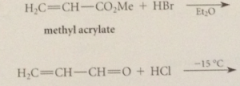
|
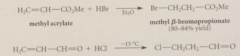
basic site of a,b-unsat carbonyl cmpd not db but carbonyl O
|
|
|
Protonation on carbonyl O folloewd by
|
rxn w halide ion
|
|
|
Electrophilic O can accept e as result of nuc rxn of halide ion @ carbonyl C or bc of conj arrangement of pi bonds at
|
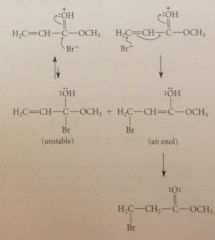
b-C
|
|
|
reaction of Br- @ carbonyl C yields
|
relatively unstable tetrahedral addition intermediate
|
|
|
rxn at B-C yields
|
enol which rapidly reverts to observed carbonyl prod
|
|
|
addition to db of a,b-unsat carbonyl cmpd is example of
|
conj addition
|
|
|
DMech of conj add of HBr similar to
|
conj addition of HBr to 1,3-butadiene (involve carbocation intermediates)
|
|
|
nucleophilic conj addition such as addition of cyanide
|
has no parallel in rxns of simple conj dienes
|
|
|
any conjugate addition rxn competes w
|
carbonyl group rxn
|
|
|
in case of aldehydes & ketones, conj addition competes w
|
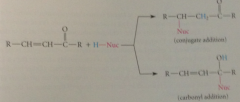
addition to carbonyl group
|
|
|
In case of estres, conj add competes w
|
nuc acyl sub
|
|
|
Relatively weak b that give reversible carbonyl-add rxns w ordinary aldehydes & ketones tend to give
|
conj add w a,b-unsat aldehydes & ketones
|
|
|
Among relatively weak bases are
|
cyanide ion, amines, thiolate ions, enolate ions derived from B-dicarbonyl cmpds
|
|
|
Conj add observed w these nucs bc
|
conj add prod are more stable than carbonyl add products
|
|
|
If carbonyl add is reversible (even if it occurs more rapidly)
|
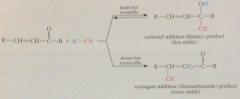
conj addition can drain the carbonyl cmpd from addition equil & conj add prod is formed ultimately
|
|
|
Conj add prod is
|
thermodynamic (more stable) prod of rxn
|
|
|
Greater stability of conj add prod
|
conj add retains C-C db @ expense of carbonyl group - C=O bond is considerably stronger than C=C bond
|
|
|
Carbonyl addition
|
kinetically favored process (faster than conj addition)
|
|
|
When nuc used that undergo irreversible carbonyl add
|
carbonyl add prod observed rather than conj add prod
|
|
|
LiAlH4 & organolithium reagents add irreversibly to carbonyl groups
|
form carbonyl addition prod whether reactant carbonyl cmpd is a,b-unsat or not
|
|
|
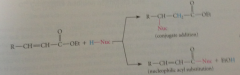
many of same nuc that undergo conj add w aldehydes & ketones also undergo
|
conj add w esters
|
|
|
stronger b that react irreversibly @ carbonyl C react w esters to give
|
nuc acyl sub prod
|
|
|
Hydroxide ion reacts w a,b-unsat ester to give
|
prod of saponification, a nuc acyl sub rxn, bc saponification is not reversible
|
|
|
LiAlH4 reduces a,b-unsat esters @ carbonyl group bc
|
rxn of hydride ion @ carbonyl group is irreversible
|
|
|
conj addition usually occurs w nuc that are relatively
|
weak bases
|
|
|
stronger bases give
|
irreversible carbonyl addition or nuc acyl sub rxns
|
|
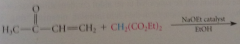
enolate ions, esp derived from malonic ester derivatives, b-keto esters & like
|
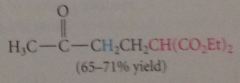
undergo conj add rxns w a,b-unsat carbonyl cmpds
|
|
|
mech follows same pattern for other nuc conj add
|
nuc is enolate ion formed in rxn of ethoxide w diethyl malonate
|
|
|
In contrast to Claisen ester cond
|
rxn requires only cat amt base
|
|
|
rxn does not rely on ionization of prod
|
to drive to completion - goes bc c-c pi bond in starting a,b-unsat carbonyl cmpd is replaced by stronger c-c sigma bond
|
|
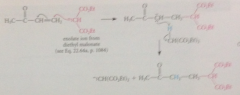
Michael additions
|
conj add of carbanions to a,b-unsat carbonyl cmpds
|
|
|
Product of a given Michael addition might originate from
|
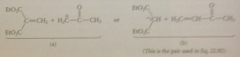
2 diff pairs of reactants
|
|
|
Weaker bases tend to give
|
conj add
|
|
|
Stronger bases tend to give
|
carbonyl group rxns
|
|
|
To maximize conjugate add
|
choose pair of reactants w less basic enolate ion
|
|
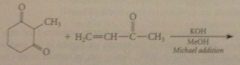
Robinson annulation
|
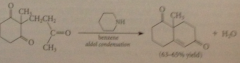
immediate prod of add subjected to aldol cond that closes a ring
|
|
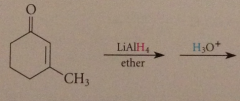
carbonyl group of a,b-unsat aldehyde or ketone like ordinary aldehyde or ketone
|
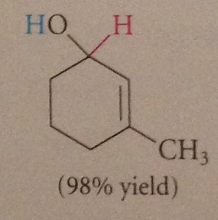
reduced to ROH w LiAlH4 - involves nuc rxn of hydride @ carbonyl C (is therefore carbonyl addition)
|
|
|
Why is carbonyl add rather than conj add observed?
|
Carbonyl add is faster than conj add & irreversible bc hydride is a poor LG
|
|
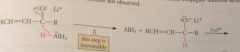
reduction of carbonyl group w LiAlH4 is
|
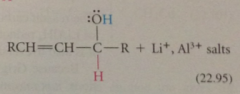
kinetically controlled
|
|
|
many a,b-unsat carbonyl cmpds are reduced by
|
NaBH4 to give mix of both carbonyl add prod & conj add prod
|
|
|
why isn't NaBH4 reduction of a,b-unsat ketones useful?
|
mixtures obtained
|
|
|
Although some cases of conj addition w LiAlH4 known
|
reagent usually reduces carbonyl groups, including carbonyl groups of esters w/o affecting db
|
|
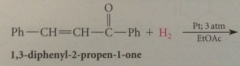
c-c db of a,b-unsat carbonyl cmpd can in most cased be reduced selectively by
|
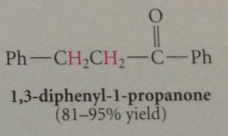
cat. hydrogenation
|
|
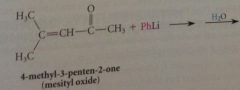
organolithium reagents react w a,b-unsat carbonyl cmpds to yield
|
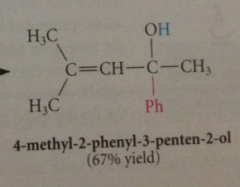
prod of carbonyl addition
|
|
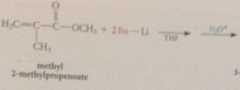
why does carbonyl add occur rather than conj addition?
|
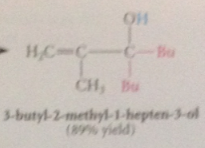
carbonyl add is more rapid than conj add & irreversible
|
|
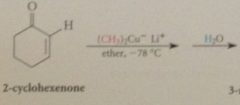
lithium dialkylcuprate reagents
|
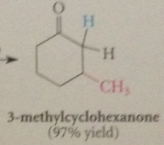
give exclusively prod of conj add when react w a,b-unsat esters & ketones
|
|
even a,b-unsat aldehydes, normally very reactive @ carbonyl group
|
give all/mostly prod of conja dd, esp at low temp
|
|
|
grignard reagents often
|
give mixtures of conj add & carbonyl add
|
|
|
bc both types add occur w grignard reagents
|
organolithium reagents used w a,b-unsat carbonyl cmpds when only carbonyl add is desired rxn
|
|
|
conj add of lithium dialkylcuprate reagents proceed by special mech promoted by presence of
|
Cu, which is esp favorable for conj add, but can be considered similar to other conj add
|
|
|
nuc rxn of an anion @ db gives
|
resonance-stabilized enolate ion
|
|
|
when h2o added to rxn mix
|
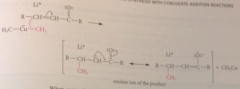
protonation of enolate ion gives conj add prod
|
|
|
grignard reagents react w a,b-unsat carbonyl cmpds to give mix of
|
carbonyl add & conj add prod
|
|
|
conj add prod due to
|
small amts transition metals known to be present in commercial magnesium
|
|
|
certain transition metals known to
|
promote conj add of grignard reagents
|
|
|
If a grignard reagent is treated w CuCl
|
magnesium organocuprate reagents are formed & give exclusively conj add like lithium counterparts
|
|
|
to carry out carbonyl add rxn w organometallic reagent
|
use organolithium reagent
|
|
|
to carry out conj add rxn
|
use lithium organocuprate
|
|
|
when is conj add rxn useful in organic synth?
|
any group @ b-position of carbonyl cmpd (or nitrile) can in principle be delivered as nuc in conj add
|
|
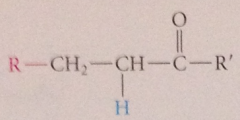
conj add can be
|
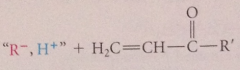
mentally reversed by subtracting nuc group from b-position of target mlc & pos fragment (usually proton) from a-position
|

