![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
126 Cards in this Set
- Front
- Back
|
aldol
|
3-hydroxybutanal / b-hydroxy aldehydes
|
|
|
aldol addition
|
rxn of 2 aldehyde mlcs to form b-hydroxy aldehyde
|
|
|
base cat aldol addition involves
|
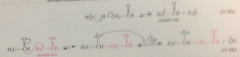
enolate ion as intermediate - enolate ion formed by rxn of acetaldehyde w aqueous NaOH adds to a 2nd mlc acetaldehyde
|
|
|
how is aldol addition like cyanohidrin formation?
|

|
|
|
is aldol addition reversible?
|
yes
|
|
|
like many other carbonyl addition rxns, equil for aldol addition is
|

more favorable for aldehydes than for ketones
|
|
|
in aldol addition rxn of acetone, equil favors
|
ketone reactant rather than addition prod, diacetone alcohol - prod can be isolated in good yield only if an apparatus used that allows prod to be removed from base cat as formed
|
|
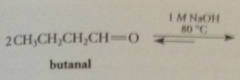
under more severe conditions (higher B conc, heat, both)
|

prod of aldol addition undergoes dehydration rxn
|
|
|
aldol condensation
|
sequence of rxns consisting of aldol addition followed by dehydration
|
|
|
condensation
|
rxn in which 2 mlcs combine to form larger mlc w elimination small mlc, often H2O
|
|
|
dehydration part of aldol condensation
|
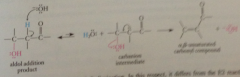
b-elimination rxn catalyzed by B, enolate ion intermediate
|
|
|
how is dehydration diff from E2
|
not concerted b-elim
|
|
|
base cat dehydration rxn of simple alcohols
|
unknown
|
|
|
do ordinary alcohols dehydrate in base
|
no
|
|
|
why do b-hydroxy aldehydes & b-hydroxy ketones dehydrate?
|
a-H are relatively acidic (B-promoted b-eliminations are fast when acidic H involved) & prod conjugated, particularly stable
|
|
|
to extent that TS of dehydration rxn resembles a,b-unsaturated ketone
|
it too is stabilized by conjugation & elim rxn accelerated (Hammond's postulate)
|
|
|
product of aldol condensation
|
a,b-unsaturated carbonyl cmpd
|
|
|
aldol condensation important method for
|
preparation a,b-unsaturated carbonyl cmpds
|
|
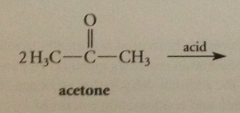
acid cat aldol condensations give
|
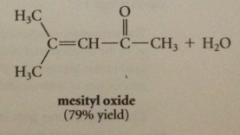
a, b unsaturated carbonyl cmpds (addition prod cannot be isolated)
|
|
|
key reactive intermediate in acid-cat aldol condensations
|
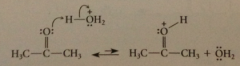
conj A of aldehyde/ ketone
|
|
|
roles of protonated ketone
|
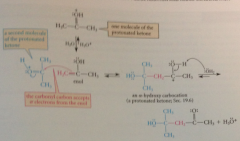
source of enol & protonated ketone is electrophilic species in the rxn - reacts as electrophile w pi e of enol to give a-hydroxy carbocation, which is also conj A of addition prod
|
|

|
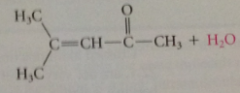
a-hydroxy carbocation loses proton to give b-hydroxy ketone prod - under acidic conditions, material spont undergoes acid-cat dehydration to give a,b-unsat carbonyl cmpd
|
|
|
aldol condensation driven to completion by
|
dehydration
|
|
|
nuc species in acid cat aldol condensation
|
enol, not enolate ion
|
|
|
enolate ions are too ___ to exist in acidic soln
|
basic
|
|
|
although enol is much less nuc than enolate ion,
|
reacts @ useful rate bc protonated carbonyl cmpd (an a-hydroxy carbocation) with whih it reacts is potent electrophile
|
|
|
nuc in base-cat aldol rxn
|
enolate ion
|
|
|
is a protonated carbonyl cmpd an intermediate
|
no - too acidic to exist in basic soln
|
|
|
electrophile that reacts w enolate ion is
|
neutral carbonyl cmpd
|
|
|
crossed aldol rxn
|
2 diff carbonyl cmpds used
|
|
|
result of crossed aldol rxn is
|
difficult-to-separate mixture
|
|
|
crossed aldol rxns that provide complex mixtures
|
are not very useful bc prod not formed in high yield & isolation of 1 prod mostly tedious
|
|
|
altho conditions that favor 1 prod or another in crossed aldol rxns have been worked out in specific cases under usual conditions (aq. or alcoholic A/B) useful crossed aldol rxns limited to situations in which
|
a ketone w a-H is condensed w an aldehyde that has no a-H
|
|
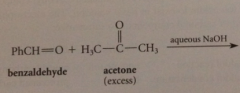
Claisen Schmidt condensation
|
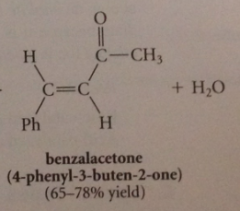
a ketone w a-H is condensed w aromatic aldehyde w no a-H
|
|
|
can addition prod be isolated?
|
no
|
|
|
condensation prod
|
most stable isomer of highly conj condensation prod
|
|
|
why can't aldehyde in Claisen-Schmidt rxn act as enolate cmpd component of aldol condensation?
|
has no a-H => 2/4 possible crossed aldol prod cannot form
|
|
|
possible side rxn doesn't occur, why?
|
aldol add rxn of ketone w itself - enolate ion from acetone can react either w another mlc of acetone or w benzaldehyde
|
|
|
add to ketone occursmore ___ than add to aldehyde
|
slowly
|
|
|
even if addition to acetone does occur
|
aldol add rxn of 2 ketones is reversible & add to aldehyde has more favorable equil constant than add to ketone
|
|
|
rate & equil for add to benzaldehyde are more __ than add to 2nd mlc acetone
|
favorable
|
|

|

|
|
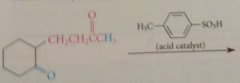
when a mlc contains 1+ aldehyde / ketone group
|
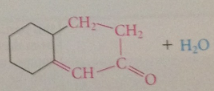
intramlclr rxn possible
|
|
|
intramlclr aldol condensations particularly favorable when
|
5/6 membered rings formed bc of proximity effect
|
|
|
questions to ask if you want to prepare particular a,b-unsat aldehyde/ ketone by aldol condensation
|
what sm required by aldol condensation? with sm, is aldol condensation of cmpds feasible?
|
|
|
determine sm for aldol condensation
|
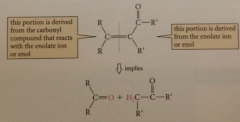
mentally split a,b unsat carbonyl cmpd
|
|
|
work backward from desired synthetic objective
|
replace db on carbonyl side by 2 H & other by carbonyl O
|
|
|
is condensation one that works, or one that is likely to give
|
troublesome mixtures
|
|
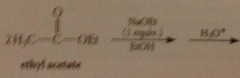
ethyl acetate undergoes Claisen condensation
|
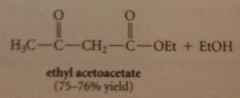
in presence of 1 equiv of sodium ethoxide in ethanol to give ethyl 3-oxobutanoate (ethyl acetoacetate)
|
|
|
b-keto ester
|
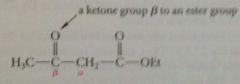
cmpd w ketone carbonyl group b to ester carbonyl group
|
|
|
Claisen condensation
|
B-promoted condensation of 2 ester mlcs to give a b-keto ester
|
|
|
first step in mech of Claisen cond
|
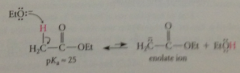
formation of enolate ion by rxn of ester w ethoxide B
|
|
|
Why is ethoxide ion used as a B w ethyl esters in Claisen cond?
|
ethoxide ion is nuc, also reacts at carbonyl group of ester to give usual nuc acyl sub rxn - products are same as reactants
|
|
|
Although ester enolate ion formed in low conc, strong B & good nuc, undergoes
|
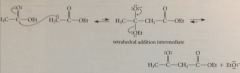
nuc acyl sub rxn w 2nd mlc ester- usual 2 step sub mech (formation of tetrahedral add intermediate followed by loss of LG)
|
|
|
overall equil lies far on side of
|
reactants: all B-keto esters are less stable than esters from which derived
|
|
|
Claisen cond must be driven to complete by applying
|
Le Chateliers
|
|
|
most common technique
|
use one full equiv ethoxide catalyst
|
|
|
In b-keto ester prod H on C adjacent to both carbonyl groups are especially
|
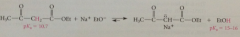
acidic & ethoxide removes one proton to form quantitatively conj B of product
|
|
|
un-ionized B-keto ester prod formed when
|
acid is added subsequently to rxn mixture
|
|
|
ethoxide ion is catalyst for rxns but
|
eventually consumed (reactant, not catalyst overall, so 1 full equiv of ethoxide must be used)
|
|
|
if Claisen condensation attempted w ester that has only 1 a-H
|
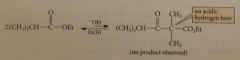
no condensation prod is formed - desired cond prod has quaternary a-C, so no a-H acidic enough to react completely w ethoxide (if prod subject to conditions of Claisen, readily decomposes back to sm bc Claisen is reversible)
|
|
|
Claisen condensation is example of
|
nuc acyl sub
|
|
|
nuc is
|
enolate ion derived from an ester
|
|
|
How is Claisen similar to saponification?
|

|
|
|
How does aldol compare to Claisen cond?
|
aldol is add of enolate ion/enol w aldehyde/ket followed by dehydration, Claisen is nuc acyl sub rxn of enolate ion w ester group, aldol cat by B & A Claisen full equiv B but no A, aldol requires 1 a-H, 2nd for dehydration step - Claisen: ester sm must have at least 2 a-H, one for each ionization
|
|
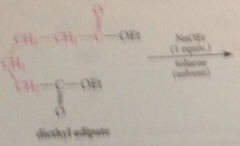
Dieckmann cond
|
intramclr Claisen cond in 5/6 membered rings
|
|
|
Dieckmann requirements
|
one full equiv of B to form enolate ion of prod & to drive the rxn to completion
|
|
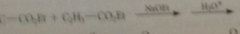
Claisen cond of 2 diff esters
|
Crossed Claisen cond
|
|
|
Not synth useful - crossed Claisen condensation of 2 esters that both have a-H
|
mixture of 4 cmpds, diff to separate
|
|
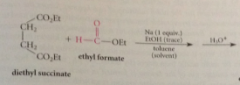
Crossed Claisen cond are useful if
|
one ester is esp reactive or has no a-H (formyl -CH=O groups readily intro w esters of formic acid such as ethyl formate) -- Formate esters fulfill both criteria for crossed Claisen cond no a-H, greater reactive carbonyl than other esters bc formate ester is part aldehyde & aldehydes particularly reactive toward nuc
|
|
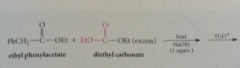
less reactive ester w/o a-H can be used if present in excess i.e. ethoxycarbonyl group w diethyl carbonate - enolate ion of ethyl phenylacetate condenses preferentially w diethyl carbonate rather than another mlc of itself bc
|
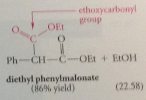
much higher conc of of diethyl carbonate & excess diethyl carbonate must then be separated form prod
|
|
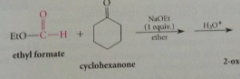
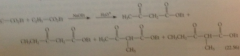
in rxn of ketones w esters, enolate ion of ketone
|
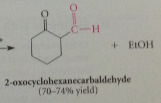
reacts @ carbonyl group of ester - enolate ion derived from ketone cyclohexane is acylated by ester ethyl formate
|
|
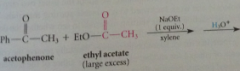
enolate ion of ketone acetophenone
|
acylated by ester
|
|
|
Several side rxns are possible but
|
do not interfere
|
|
|
Possible side rxn of cyclohexanone w itself
|
equil for aldol add of 2 ketones favors reactants, whereas Claisen cond is irreversible bc 1 equiv of B used to form enolate ion of prod
|
|
|
Ester cannot condense w itself bc
|
no a-H
|
|
|
Ester has a-H but self-condensation isn't a side prod bc
|
ketones are far more acidic than esters => enolate ion of ketone formed in greater conc than enolate ion of ester
|
|
|
ketone enolate ion can react w another mlc of ketone (unfavorable equil) OR
|
intercepted by excess of ethyl acetate to give observed prod, B-diketone
|
|
|
even tho esters less reactive than ketones, B-diketone is esp acidic (like B-keto ester) &
|
ionized compeltely by one equiv NaOEt => B-diketone formation observed bc ionization makes irreversible rxn
|
|
|
Planning synth of B-dicarbonyl cmpd
|
examine target mlc, work backward to reasonable sm, analyze rxn of sm to see whether desired rxn is reasonable or other rxns will occur instead
|
|
|
to determine sm for Claisen cond
|
mentally reverse cond by adding elements of ethanol (or another ROH) across either C-C bonds btwn carbonyl groups
|
|
|
Because there are 2 such bonds, we will generally find 2 possible
|
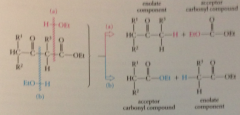
disconnections & 2 corresponding sets of sm
|
|
|
analyzing B-diketone
|

|
|
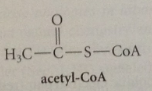
sm for biosynth of fatty acids
|
thiol ester of acetic acid called acetyl-CoA - complex functionality in mlc required for its recognition by enzymes but has no direct role in chem transformations
|
|
|
In biosynth of fatty acids, acetyl CoA converted into
|
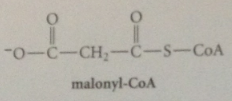
malonyl Co-A by carboxylation of a-C
|
|
|
-SCoA group in both acetyl & malonyl CoA replaced in nuc acyl sub rxn by
|
-SR: acyl carrier protein
|
|
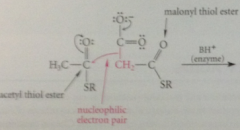
In rxn closely resembling Claisen cond, malonyl & acetyl thiol esters react in enzyme catalyzed rxn to give
|
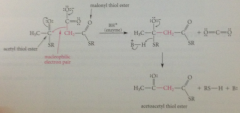
acetoacetyl thiol ester
|
|
|
nuc e pair made available by loss of
|
CO2 from malonyl CoA, which drives Claisen cond to completion
|
|
|
In laboratory, Claisen cond driven to completion by
|
ionization of prod w strong B like ethoxide
|
|
|
Strong base cannot be used in living cells
|
all rxns must occur near neutral pH
|
|
|
acetoacetyl thiol ester then undergoes successively
|
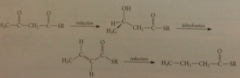
carbonyl reduction, dehydration, & db reduction, each cat by enzyme
|
|
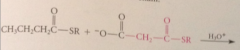
net result is
|
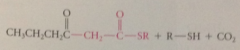
acetyl thiol ester converted into a thiol ester w 2 additional C
|
|

|
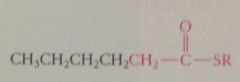
sequence of rxns repeated, adding another 2 C to chain
|
|
|
4 rxns repeated w 2 C to the C chain @ each cycle until
|
fatty aid w proper chain length obtained
|
|
|
fatty acid thiol ester then
|
transesterified by glycerol to form fats & phospholipids
|
|
|
why do common fatty acids have an even # C atoms?
|
They are formed from successive addition of 2 C acetate units
|
|
|
What other cmpds in nature are synth from acetyl CoA?
|
isopentenyl pyrophosphate, basic building block of isoprenoids & steroids + some aromatic cmpds found in nature
|
|
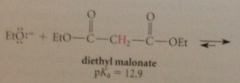
malonic ester synth
|
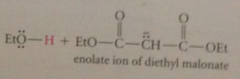
like other b-dicarbonyl cmpds has unusually acidic a-H so conj B enolate ion can be formed w alkoxide bases like Na ethoxide
|
|
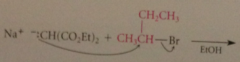
conj B anion of diethyl malonate is nuc & reacts w alkyl halides & sulfonate esters in sn2 rxns
|
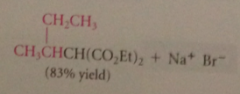
can be used to intro alkyl group @ a position of malonic ester - even secondary halides - can be extended to prep of CA
|
|
|
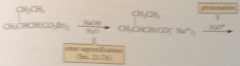
saponification of diester & acidification of resulting soln gives
|
substituted malonic acid derivative
|
|
|
Heating any malonic acid derivative causes it to
|
decarboxylate
|
|
Result of alkylation, saponification, & decarboxylation
|
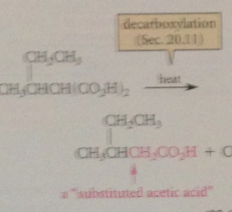
CA that is conceptually a substituted acetic acid - acetic acid mlc w alkyl group on a-C
|
|
|
malonic ester synth
|
overall sequence of ionization, alkylation, saponification & decarboxylation starting from diethyl malonate
|
|
|
Alkylation step of malonic ester synth results in
|
formation of new C-C bond
|
|
|
Anion of malonic ester can be alkylated twice in 2 successive rxns w
|

diff alkyl halides to give after hydrolysis & decarboxylation a disubstituted acetic acid
|
|
|
If alkyl halides R-X & R'-X are among those that undergo Sn2 rxn, target CA can be
|
prepped by malonic ester synth
|
|
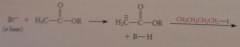
why not avoid wasting a Co2Et group in synth of CA by malonic ester alkylation by directly alkylating the enolate ion of an acetic acid ester?
|
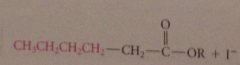
enolate ions derived from esters, once formed, undergo another faster rxn: Claisen cond w parent ester
|
|
|
What can be used to form stable enolate ions rapidly at -78 C from esters?
|
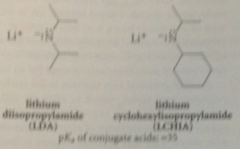
very strong, highly branched N bases
|
|
|
Amide
|
conj B anion of an amine - has double usage as CA derivative
|
|
|
Because esters have pKa values near 25, amide B are strong enough to
|
convert esters completely into their conj B enolate ions
|
|
|
Ester enolate anions formed w B can be
|
alkylated directly w alkyl halides
|
|
|
Esters w quaternary a-C atoms can be prepared by this method but not
|
malonic ester synth
|
|

|

|
|
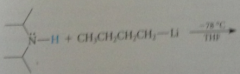
N bases gen from corresponding
|
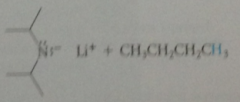
amines & butyllithium at -78C in THF solvent
|
|
|
ester alkylation is considerably more
|
expensive than malonic ester synth & requires special inert atm techniques bc strong B used react vigorously w O2 & H2O
|
|
|
Malonic ester synth useful for
|
large scale syntheses
|
|
|
Prep of lab samples or cmpds unavailable from mal ester synth
|
prep & alkylation of enolate ions w amide B particularly valuable
|
|
|
Why does use of strong amide B avoid Claisen cond?
|
reaction is run by adding ester to B
|
|
|
When a mlc of ester enters the soln
|
can react w strong B to form enolate ion or w mlc of already formed enolate ion in Claisen cond
|
|
|
Rxn of esters w strong amide B so much faster at -78C than Claisen cond that
|
enolate ion is formed instantly & never has chance to undergo the Claisen cond
|
|
|
Claisen cond is avoided bc
|
ester & enolate ion are never present simultaneously except for an instant in rxn flask
|
|
|
Potential side rxn
|
uc rxn of amide base or conj A amine at ester carbonyl group
|
|
|
Amines react w esters to give prod of aminolysis but conj B of amines, strong B, don't react more rapidly w esters bc
|
competition - when an amide base reacts w the ester, can either remove a proton or react at carbonyl C
|
|
|
Rxn at carbonyl C retarded by
|
VDW repulsions btwn groups on carbonyl cmpd & large branched groups on B
|
|
|
If amide b could be in contact w ester long enough would react @ carbonyl C but B reacts more rapidly by --
|
abstracting a-proton
|
|
|
Reaction w tiny H does not involve VDW that would occur if B were to react @ carbonyl C, so
|
amide B takes path of least resistance: forms enolate ion
|

