![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
153 Cards in this Set
- Front
- Back
|
What is a compound nerve action potential recording (CNAP)?
|
A compound AP i.e. it is the sum of extracellular signs of a propagating AP
Brief electrical pulse used to trigger action potential in the fibres of a peripheral nerve trunk Amplifier/recording device tracks electrical potential differences with time after nerve stimulation CNAP can be recorded from a second set of electrodes further along the nerve |
|
|
What is a compound muscle action potential recording (CMAP)?
|
A compound AP i.e. it is the sum of extracellular signs of all muscle fibres activated in muscle
Stimulate n. --> all muscle fibres will be activated in healthy person (ie NMJ working properly) |
|
|
Why would you record the compound muscle action potential (CMAP)?
|
To assess potential motor nerve damage, or a problem with the NMJ.
|
|
|
What would a reduced amplitude in a compound muscle action potential recording indicate?
|
Something wrong with NEUROMUSCULAR TRANSMISSION
|
|
|
What would a time lag in a compound muscle action potential recording indicate?
|
may indicate something wrong with propagation down motoneuron
|
|
|
In a patient with Myasthenia gravis, what would you expect their compound muscle action potential recording to look like?
|
Repetitive stimulation may reveal fatiguing CMAP.
Would see a decline in the CMAP amplitude |
|
|
In a compound nerve action potential recording, what would a time lag indicate?
|
Something wrong with myelination of the nerve.
|
|
|
In a compound nerve action potential recording, what would a change in shape of the curve indicate?
|
may reveal altered proportions of different classes of nerve fibres (differing in their extent of myelination and therefore in their rates of conduction)
|
|
|
CMAP (compound muscle AP) is the same as what?
|
EMG - electromyography
|
|
|
T/F: voltage gated Ca2+ channels on the post-synaptic membrane open in response to depolarisation
|
FALSE - the channels are on the PRE-SYNAPTIC membrane!
|
|
|
Describe what happens at the NMJ on arrival of an AP
|
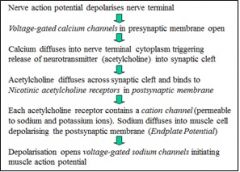
|
|
|
T/F: A "Primary Gutter" is where the muscle fibre surface folds inwards to receive nerve terminal
|
TRUE
|
|
|
T/F: The pre-synaptic membrane at the NMJ forms “secondary” post-junctional folds
|
FALSE - it is the post-synaptic membrane that does this.
|
|
|
What is a MEPP?
|
Mini endplate potential as a result of a rare, spontaneous exocytosis of a vesicle load (quantum) of ACh
Small amplitude, spontaneous postsynaptic depolarization events |
|
|
What is an EPP?
|
Endplate potential.
Normally has a large amplitude (~70mV) and is produced by simultaneous release of many quanta of ACh acting on huge numbers of postsynaptic ACh receptors |
|
|
What coordinates the release of quanta of ACh?
|
Influx of CALCIUM.
[which depends upon voltage-gated calcium channels in the nerve terminal and the relatively high extracellular concentration of calcium ions] |
|
|
T/F: MEPPs can be seen even when the muscle is at rest?
|
True!
|
|
|
Normal distribution of MEPP amplitude suggests what?
|
This is a consistent phenomenon.
Characteristic amplitude suggests fixed quantity of ACh (quantum) |
|
|
T/F: Reducing extracellular calcium concentration can cause failure of the muscle AP
|
True - if reduce below physiological levels of approx 1mM.
Revealed sub-threshold EPP that would normally have caused an AP |
|
|
T/F: Release of ACh can occur in multiples of the quantum
|
True
|
|
|
T/F: Upon arrival of the AP at the NMJ, all release sites release all their ACh
|
False.
The release of ACh is probabilistic - don't release every time. Also, not all release sites release ACh (always a reserve of vesicles that haven't unloaded yet) |
|
|
T/F: ACh is released simultaneously from each contributing release site on the pre-synaptic membrane
|
True.
So that Na+ goes into the post-synaptic cell at the same time |
|
|
What is Lambert-Eaton Myasthenic syndrome? What are the consequences?
|
Autoimmune antibodies attack presynaptic voltage gated calcium channels.
Reduced calcium influx into nerve terminal (due to disrupted Ca channels) Fewer quanta of ACh released each time n. depolarised Smaller amplitude EPP Failure to reach threshold --> no muscle action potential |
|
|
What is Myasthenia gravis?
|
Autoimmune antibodies against the postsynaptic ACh receptor impair the response of the postsynaptic membrane to ACh.
Can impair alignment of release sites to binding sites Reduced EPP and similarly reduced MEPP amplitude [Note can also have antibodies against MuSK] |
|
|
What is the "fatiguing phenomenon" in regards to the NMJ?
Why is this relevant when discussing Myasthenia gravis? |
The no. of quanta released each time goes down during train of stimuli [i.e. tetanus].
High frequency trains of impulses deplete the no. of available vesicles docked on the membrane ready to go. This is normally not a problem because the quantal amplitude is more than enough In MG – have a reduced quantal amplitude so fatigue more easily (nerves can’t keep up) |
|
|
Why don't nerve terminals at the NMJ release all their ACh?
|
Nerve terminals function by having more vesicles available for release than they need
The probability of release is approx. 1/10 You need these vesicles that are already there because it takes too long to recycle |
|
|
The EPP amplitude depends on what?
|
Quantal amplitude
No. of quanta released |
|
|
What are some treatments for Myasthenia gravis?
|
Immunosuppressants
Thymectomy Plasma exchange or plasmapheresis Cholinesterase inhibitors |
|
|
How can cholinesterase inhibitors help in Myasthenia gravis?
|
AChE (acetylcholinesterase) hangs out in the synapse to 'mop up' ACh after its been released.
If you block it, then there is more ACh hanging around for longer - Incr. EPP which can now reach threshold |
|
|
Skeletal muscle accounts for what proportion of body weight?
|
40%
It is the largets 'tissue' in the body |
|
|
Rapid transmission along a nerve relies on what?
|
Myelination
Large diameter |
|
|
What are the 4 classes of mechanoreceptors in human skin?
What are their relative receptive fields? |
Pacinian Corpuscles - large
Meissner’s Corpuscles - small Merkel-cell-neurite complex - small Rufini organ - spindle shaped |
|
|
When do Pacinian Corpuscles respond ?
|
Brief response when stimulus applied or removed
|
|
|
When do Meissner's Corpuscles?
|
Brief response when stimulus applied [touch sensitivity]
|
|
|
When do Merkel-cell-neurite complexes respond?
|
Throughout duration of stimulus
|
|
|
To what do Ruffini organs respond?
|
Skin stretch
|
|
|
Which mechanoreceptors contribute to discriminative touch and accurate localisation?
|
Merkel-cell-neurite complex
Meissner's Corpuscles [both have SMALL receptive fields] |
|
|
Where do signals from peripheral receptors which contribute to perception and sensation go?
|
To the spinal cord - thalamus - cortex
[NB: Some fibres synapse in SC and brainstem (--> unconscious reflexes)] |
|
|
What is isotonic vs. isometric contraction?
|
Isotonic = muscle shortens against constant load (i.e. muscle does WORK)
Isometric = muscle prevented from shortening |
|
|
What kind of cell is a muscle fibre?
|
Multinucleate giant cell
|
|
|
T/F: Skeletal muscle will not contract until there is an efferent command from the CNS via motor neurons
|
TRUE
|
|
|
What is a "motor unit"?
|
Motoneuron plus the subset of muscle fibres it innervates
|
|
|
Recruiting more motor units will lead to what?
|
Greater force output from the muscle
|
|
|
Describe the contractile machinery of the muscle fibre.
|
Each muscle fibre - packed with 1000s of myofibrils.
Each myofibril - repeating sarcomeres. Thin filaments = polymerised actin and regulatory proteins (troponin and tropomyosin) Thick filaments = myosin. Thick filaments have 'projections' (cross bridges) that swing out, interacting with actin to produce relative motion (i.e. contraction) |
|
|
Thin filaments are held together by what?
|
Z disc
|
|
|
What is the length of an average sarcomere?
|
2-3um
|
|
|
What is the sliding filament theory?
|
The shortening of a sarcomere via interaction between actin and myosin (cross bridging).
Power stroke generates force and relative motion between the thick and thin filaments |
|
|
Describe the metabolic machinery of the myofibril.
|
There are enzyme systems and substrates around each myofibril responsible for regeneration of ATP (either aerobically or anaerobically).
In active muscle, elevated cytosolic Ca2+ triggers GLYCOGEN BREAKDOWN --> ATP |
|
|
How does elevated cytosolic Ca2+ in active muscle regenerate ATP?
|
1. Ca2+ binds calmodulin
2. Activates phosphorylase kinase 3. Activates glycogen phosphorylase 4. Glycolysis initiatied 5. Oxiative phosphoylation to replenish ATP |
|
|
Which 2 systems of tubules ensleeve the myofibril? What are their functions and how do they interact with each other?
|
Sarcoplasmic reticulum - Ca2+ storage
T tubules - transverse tubules deliver excitation to the whole muscle T tubules form close contacts with the SR - so that the SR releases Ca2+ at the 'doorstep' of each sarcomere |
|
|
What are the "switching machinery" of the myofibril
|
1. T tubules - electically excitable invaginations of the sarcolemma
2. Sarcoplasmic reticulum - stores Ca2+ 3. Regulatory proteins that inhibit myosin and actin interaction (keep m. relaxed) |
|
|
What causes relaxation of a muscle?
|
Sequestration of Ca2+ back into the sarcoplasmic reticulum
|
|
|
What is the Functional significance of the myofibrillar organisation?
|
Large scale integration of the small molecular forces and movements into macroscopically useful forces and movements.
o Without this order, energy from ATP hydrolysis would result in disordered molecular motion or heat Rapid diffusion of Ca2+ and metabolites in and out of contractile machinery (->rapid initiation and termination of contraction |
|
|
How are myofibrils organised?
|
Almost crystalline organisation of the contractile proteins.
|
|
|
At rest, how is intracellular [Ca2+] maintained in the muscle fibre?
|
Active pumping from cytosol to the SR by CaATPase
--> relaxes the contractile machinery In SR, Ca2+ stored by binding to calsequestrin |
|
|
What blocks the myosin binding site at rest?
What removes it? |
Tropomyosin
[NB: each tropomyosin covers the binding site on 7 actin monomers] Removal: binding of Ca2+ to toponin causes tropomyosin to roll away ["Steric block hypothesis"] |
|
|
What happens when the T tubules are depolarised?
|
1. Conformational change in DHP-receptors
2. Ca2+ release channels open 3. Ca2+ diffuses out of SR ["calcium transient"] 4. Ca2+ binds troponin 5. Tropomyosin moves, leaving the myosin binding site free |
|
|
Outline one cross-bridge cycle
|
1. ATTACHMENT
Myosin binds to the myosin binding site on actin (since tropomyosin has been removed) 2. POWER STROKE Myosin spontaneously undergoes bending movement (pulling actin towards centre of thick filament). At same time, releases MgADP and Pi. [In the resting state, myosin charged with free energy of MgATP] 3. DISSOCIATION MgADP binds cross-bridge and displaces actin |
|
|
What are DHP receptors and where are they found?
|
Dihydropyridine (DHP) receptors are VOLTAGE SENSORS.
They are found on T tubules at points of contact with the SR --> i.e. they have contact with the Ca2+ release channels on the SR membrane |
|
|
T/F: Cross bridge cycling will continue as long as there is raised cytosolic [Ca2+]
|
True blue!!!
|
|
|
Compare a 'twitch' with tetanus.
|
Twitch - transient mechanical response of the muscle to a single stimulation
Tetanus - prolonged contraction of a muscle in response to a rapid train of action potentials [transient and mechanical oscillations tend to fuse] |
|
|
T/F: Maximal tetanic tension may be 3-10x higher than maximal tension of twitch
|
TRUE!
|
|
|
T/F: Work is done during both isometric and isotonic contraction.
|
FALSE.
Work is NOT DONE during isometric contraction - the load is too much for the muscle to move |
|
|
What is the "Force-Velocity" relation in isotonic muscle contraction?
|
The velocity of shortening is INVERSELY related to the force against which a muscle shortens
|
|
|
At what proportion of a muscle's maximal force do we get maximal power?
|
30%
[Maximal power developed at load equivalent to 30% maximal force of muscle] |
|
|
The greater the overlap of thin and thick filaments, the greater the ____
|
FORCE
|
|
|
T/F: There are different fibre types within a motor unit
|
False.
Muscle fibres of a given motor unit are of the same fibre type |
|
|
Which muscle fibre type is most resistant to FATIGUE?
|
Slow (type I)
|
|
|
Which muscle fibre type(s) have
1. Many mitochondria 2. Many capillaries 3. High myosin ATPase activity 4. Oxidative phosphorylation capacity |
1. Many mitochondria
--> Type I, Type IIa 2. Many capillaries --> Type I, Type IIa 3. High myosin ATPase activity --> Type IIa, Type IIx (i.e. fast fibres) 4. Oxidative phosphorylation capacity --> Type I, Type IIa (i.e. those that undergo oxidative metabolism) |
|
|
How do Type IIX fibres generate ATP?
|
Anaerobically via glycolysis
[get lactic acid buildup] |
|
|
What is the tension of each muscle fibre?
|
Type I- Low
Type IIa - Intermediate Type IIX - High |
|
|
What are the functions of the following fibres:
- Type I - Type IIa - Type IIX |
Type I = slow movements, posture
Type IIa = intermediate movements Type IIX - sudden spurts of activity |
|
|
What size motor unit would you used for refined movements?
|
Small
|
|
|
As demand on a muscle increases, what size motor units would you recruit?
|
Start off by recruiting smaller ones, then as demands increase start to recruit progressively larger units
|
|
|
Intense training leads to fibre type transformation from what to what?
|
from IIX --> IIa --> I
|
|
|
Intense, regular exercise stimulates hypertrophy by causing replication of what cells?
|
Satellite cells
|
|
|
T/F: Intense, regular exercise decreases protein synthesis and metabolic enzymes
|
FALSE. Increases both
|
|
|
What is the significance of increased metabolic enzymes after intense, regular training of muscles?
|
More resistant to fatigue
|
|
|
What are the consequences of muscle disuse?
[Can these changes affect systemic body metabolism?] |
atrophy (increased protein degredation, loss of myofibrils, red uced fibre X-sectional area) and fibre type changes
[Yes] |
|
|
What are the consequences of the following (w.r.t. muscle)
1. Partial denervation 2. Complete denervation 3. Chronic denervation |
1. Partial denervation
--> muscle weakness 2. Complete denervation --> muscle paralysis 3. Chronic denervation --> profound muscle wasting, fibrillations |
|
|
What are satellite cells and what do they do?
|
Satellite cells = reserve myoblasts b/w basal lamina and sarcolemma of each muscle fibre.
They are stimulated to replicate + fuse with parent fibre to support muscle growth in response to GH [puberty] and support muscle hypertrophy [exercise/training] |
|
|
T/F: Muscle fibres have great regenerative capacity post-injury
|
TRUE.
Necrotic tissue removed by macrophages (leaving satellite cells within basal lamina) |
|
|
How do injured muscle fibres get repaired?
|
Injured tissues release GFs that stimulate replication of satellite cells, initiating regenerative myogeneis by undergoing fusion to form myotubes.
Myotubes mature into new muscle fibres. |
|
|
T/F: Musculoskeletal pain = one of most common medical complaints and known to have some relationship to work
|
true
|
|
|
Most MSK conditions that are thought to be related to workplace involve which body regions?
|
back, neck or upper limbs.
[Most common = lower back (manual handling, vibration, etc) and upper limb inflammatory disorders (repetitive and/or awkward movements)] |
|
|
In 2000, what percentage of people had experienced an injury in the workplace in the past year?
|
5% people 15+
|
|
|
Occupational overuse syndrome (OOS), is associated with what?
|
- Lack of autonomy at work
- Incr. work stress - Depression - Problems with inter-personal relationships |
|
|
What are the risk factors for Carpal Tunnel Syndrome?
|
o Female gender
o Menopausal age o Obesity o Lack of fitness o Diabetes or positive family Hx of diabetes o OA of MCP joint of thumb o Smoking o Lifetime alcohol intake |
|
|
T/F: In most cases of work-related MSK pain, patients will have better LT outcome if return to work ASAP
|
TRUE
|
|
|
What is the incidence in of carpal tunnel syndrome?
|
Occurs in 6% females.
[10x more likely in females] |
|
|
What are the symptoms of carpal tunnel syndrome?
|
Numbness in median n. distribution of hand
Pain and paraesthesiae often felt outside median n. territory (even up to shoulder) Sensory disturbance can produce difficulty w/ fine manipulative skills [in severe cases this may also be due to thenar muscle weakness] Paraesthesiae and pain characteristically worse at night Patients wake up from sleep in early hours of morning with numbness, paraesthesiae and pain [discomfort, pain may radiate to shoulder] Symptoms may be relieved by shaking/exercising arm or hanging over edge of bed [relief by shaking hand important diagnostic clue – would normally hurt if other upper limb discomfort] Symptoms often ass’d with periods of strenuous activity of hand or maintained grip, esp if wrist maintained in mild extension [eg driving car] |
|
|
What disorders could precipitate carpal tunnel syndrome?
|
Those that:
Decrease space in carpal tunnel, OR Increase nerve susceptibility to pressure |
|
|
What are some differential diagnoses of CTS?
|
C6 Compression
Cervical rib compression C8/T1 n. roots Peripheral neuropathy Flexor tenosynovitis or tendonitis RSI |
|
|
When is surgical treatment indicated in CTS?
|
Acute carpal tunnel compression with progressive impairment of median n. fn
Most commonly in chronic compression where symptoms persist despite conservative measures Signs of sensory loss and/or motor weakness and wasting |
|
|
What is "myotonia"
|
Failure to relax at the end of a voluntary contraction.
|
|
|
What is "malignant hyperthermia"?
|
Rare disease triggered by anaesthesia (which abnormally opens SR Ca2+ release channels)
Simultaneous release of Ca2+ in all muscle causes contracture and RAISED TEMP. [blocked by dantrolene] |
|
|
Each cross bridge cycle consumes how many molecules of ATP?
|
one
|
|
|
What are the sources of ATP in muscle? For how long can these sources provide ATP?
|
1. ATP (lasts 6s)
2. Phosphocreatine (lasts 20s) 3. Anaerobic glycolysis (produces lactic acid, lasts 1-2 min) 4. Aerobic, oxidative metabolism of glycogen (lasts 1-2 hrs) |
|
|
What is McArdles disease?
|
Lack the enzyme that breaks down glycogen.
Get rapid, painful fatigue. Gentle exercise OK - can perform slow oxidative phosphorylation on fatty acids/glucose |
|
|
What happens in very prolonged ischaemia to muscles?
|
ATP consumed by resting metabolic processes.
Cycling cross bridges can no longer detach - the muscles become very stiff to stretch (=rigor mortis) |
|
|
T/F: Both cardiac and skeletal muscle can survive several hours of ischaemia
|
FALSE - cardiac muscle CANNOT survive this long, nor does it have the extensive repair mechanisms of skeletal m.
|
|
|
What is muscle atrophy?
|
Same no of cells.
Muscles smaller, thinner. Less contractile proteins. |
|
|
What does endurance training such as marathon running do to muscle fibres?
|
Causes them to convert to SLOW FIBRES which are more aerobic and resistant to fatigue
|
|
|
What triggers muscle hypertrophy?
|
Small number of maximal contractions
|
|
|
What is the Zone of Polarising Activity?
|
At the posterior margin of the limb bud there is a small group of cells known as the zone of polarising activity (ZPA). These cells produce the protein ‘sonic hedgehog (shh)’ which sets up a gradient (posterior to anterior) across the limb bud.
|
|
|
How is the forelimb specified cf. hindlimb during development?
|
Forelimb bud mesenchyme expresses Tbx5
Hindlimb bud mesenchyme expresses Tbx4 |
|
|
When does bone mesoderm condense into cartilage
|
wk 5 to 8.
|
|
|
When does fetal movement first occur?
When is it felt by mother? |
Spontaneous 8-10 weeks. Felt by mother 16-20 wks
Necessary for normal development |
|
|
At what point has the fetus grown to the limit of uterine cavity?
|
35-38 weeks fetus grown to limit of uterine cavity and relative proportion of amniotic fluid decreases causing further constraint.
|
|
|
What percentage of babies are born with extrinsic deformity?
What proportion of these will spontaneosly resolve? |
2% of babies are born with extrinsic deformity. 90% resolve normally
|
|
|
What is amniotic fluid?
|
Fluid that surrounds the fetus - cushion and movement without constraint
essential for lung development |
|
|
What is congenital hip dysplasia?
What increases the risk? |
Congenital hip dysplasia a condition in which the hip joint is unstable and easily dislocated at birth. There is a familial tendency.
Anything that causes crowding within the uterus incr. the risk (e.g. breach position, big baby) |
|
|
During development, when do cells become "determined" to form a particular part of adult limb
|
Over a short period lasting from about day 26 to day 33 all the cells in
the limb bud become “determined” to form a particular part of the adult limb |
|
|
What are the 3 axes in the limb bud.
|
1. PROXIMODISTAL
signal comes from the apical ectodermal ridge and involves FGF-8 2. ANTEROPOSTERIOR signal comes from zone of polarising activity (ZPA) and involves sonic hedgehog (iii) DORSOVENTRAL signal from dorsal ectoderm – Wnt-7a and a signal from the ventral ectoderm En-1 |
|
|
The majority of radiopharmaceuticals are bound to what substance ?
|
Tc-99m
|
|
|
What are radiopharmaceuticals?
|
Radioisotope bound to a pharmaceutical.
Radioisotope emits gamma rays for detection, while the pharmaceutical determines the biological distribution |
|
|
Why use SPECT (Single Photon Emission Computed Tomography)?
|
Gives depth information and removes superimposition seen on planar imaging
3 planes - coronal, saggital, transverse Improves image contrast and allows better localization of lesions |
|
|
What radiopharmaceutical may we use in a bone scan? why?
[what does uptake suggest] |
Tc-99m - methylenediphosphonate
Phosphonates become adsorbed on the hydroxyapatite extracellular matrix of bone [uptake suggests bony metabolism i.e. osteoblastic activity] |
|
|
What are the 3 phases of a blood scan? what are they each looking for?
|
1. BLOOD FLOW (60s)
2. BLOOD POOL (1-5min) Assessing tissue vascularity (assessing infection, trauma, inflammation, ...) 3. DELAYED (2-4hr) Osteoblastic activity |
|
|
T/F: Almost all pathological processes in bone are associated with altered bony metabolism
|
true
|
|
|
Why combine SPECT with CT?
|
Because CT will give good anatomical detail for targeted treatment of any functional abnormality found on SPECT
|
|
|
What activity do you see in bony metastases?
|
Tumour activates osteoclasts --> bony lysis --> activates osteoblasts --> sclerosis
We see bony lysis or sclerosis. NOTE - bone scan is MORE SENSITIVE if you have lysis and sclerosis going on. If it's just LYSIS then it's LESS SENSITIVE |
|
|
T/F: In otherwise normal bone, 3 phase bone scans are highly sensitive and specific for osteomyelitis
|
TRUE
With underlying osseous abnormalities, the specificity is reduced |
|
|
With underlying osseus abnormalities, how would you pick up osteomyelitis?
|
Additional gallium or labelled WBC scintigraphy greatly improves specificity
|
|
|
What substance is used for bone infection imaging?
Why? |
GALLIUM-67
Taken up into inflammatory and malignant tissues [cells which have a transferrin-R since Gallium-67 is transported by transferrin] |
|
|
How could one assess infection in peripheral bones?
|
Radioactively labelled WBC.
[better in acute cf. chronic osteomyelitis] |
|
|
What are osteoprogenitor cells?
|
derived from mesechymal stem cells and give rise to osteoblasts
|
|
|
Is there active bone growth on the bone surface?
|
No. This is where the bone lining cells are.
They regulate the movement of calcium and phosphate into and out of the bone |
|
|
What are OSTEOCLASTS
|
phagocytic cells derived from haemopoietic progenitor cells in the bone marrow
|
|
|
How do you recognise the following in a bone section?
1. Osteoblasts 2. Osteocytes 3. Osteoclasts 4. Bone-lining cells |
1. Osteoblasts
Cuboidal and aggregate into a single layer of cells lying in apposition to forming bone. 2. Osteocytes Small cells located in the lacuna 3. Osteoclasts Large, multi-nucleated cells that sit directly on the bone being removed 4. Bone-lining cells Flat cells and attenuated cytoplasm located on the bone surface |
|
|
What is a Howship’s lacuna?
|
shallow bay in bone created by the osteoclast’s resorption activity
|
|
Label
|
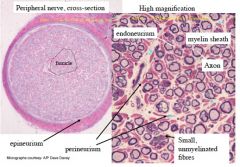
|
|
|
What is the typical resting membrane potential ?
What does this potential depend on? |
Vm = -60 mV
Depends on the concentration gradients established by the Na-K-ATPase pump [which in turn depends on ENERGY supply] |
|
|
If the Na-K-ATPase pump is blocked, for how long will APs continue to fire?
What can be inferred from this? |
Tens of minutes
the sodium/potassium pump does not directly generate nerve signals, rather it is needed mainly to sustain the concentration gradients over the long term |
|
|
What are the chemical driving forces in the resting cell for Na+ and K+
|
K+ wants to diffuse OUT of the cell (higher concn inside)
Na+ wants to diffuse INTO the cell (high concn outside) |
|
|
At rest, to what ion is the membrane more permeable? by how much?
|
20x more permeable to POTASSIUM (cf. sodium)
|
|
|
What is the Nernst Potential for K+?
|
Ek = -75mV
If potassium alone was diffusing, eventually the electrical driving force that it generates would cancel out its outward chemical driving force. This occurs at the Equilibrium Potential or Nernst Potential for K+ |
|
|
Why is the Nernst potential for Na+ positive??
|
Ena = +55mV
Because of the direction of the concentration gradient (high out, low in) |
|
|
T/F: Permeability to K+ is greater than for Na+ but net force on K+ is smaller
|
TRUE
|
|
|
Why is the resting membrane potential so close to the Nernst Potential for K+ and not Na+???
|
Because membrane is 20x more permeable to K+
[that's why the membrane potential rises when more sodium channels open, bc you are increasing the permeability of Na] |
|
|
What is the Hodgkin cycle?
|
a self-regenerating opening of voltage-gated sodium channels
|
|
|
Continuous propagation occurs in what types of axons?
|
UNMYELINATED
|
|
|
Where is the Hogkin cycle initiated?
|
at the TRIGGER ZONE on a neuron (this is where voltage gated Na+ channels are most concentrated)
|
|
|
T/F: Schwann cells enwrap ALL peripheral neurons
|
TRUE - provide nutrients and other support
-->loosely wrap around small diameter unmyelinated fibres -->many layers of tightly wrapped schwann cells around large diameter, myelinated fibres |
|
|
Which axons are the largest?
|
Muscle sensory afferent fibres
Motor neurons |
|
|
Where are voltage gated Na channels concentrated on myelinated axons?
|
Axon hillock
Nodes of Ranvier |
|
|
T/F: AP signal is amplified at each Node of Ranvier by Hodgkin cycling
|
TRUE
|
|
|
How does the signal between nodes of Ranvier passively travel?
|
As local circuit current between nodes.
Internodes (i.e. myelin) insulate and allow these local circuit currents to spread faster and further |
|
|
What are some consequences of demyelination?
|
Incr. permeability of membrane to ions
Incr. membrane capacitance in demyelinated internode regions Decr. membrane electrical resistance (-->loss of amplitude) Slowing of AP propagation |
|
|
Why do nerves need blood supply?
|
to maintain sodium/potassium pump function (-->concentration gradients and action potential propagation)
|
|
|
What is 1st degree damage of a nerve?
|
1st degree damage involves temporary block of
propagation without axon damage- eg external compression on blood vessels |
|
|
What is 1st degree damage of a nerve?
|
Part of the nerve fibre distal to the injury degenerates as distal stump no longer receives anterogradely transported materials.
[Proximal stump usually sprouts a new growth cone, but reinnervation will take a very long time] |
|
|
What is involved in 3rd-5th degree damage of a nerve?
|
Loss of endo- and/or peri- and epineurium deprives
axon growth cones of guidance cues and support [5th degree-reinnervation impossible] |

