![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
|
Causes of Cell injury |
hypoxia ischemia physical agents drugs and chemicals infectious agents immunologic reactions nutritional imbalances genetic |
|
|
hypoxia |
decrease in amount of oxygen to cell impairs oxidative respiration |
|
|
ischemia |
inadequate blood supply to cells/tissue most common cause of hypoxia |
|
|
physical agents |
trauma burns deep cold |
|
|
immunologic reactions |
anaphylaxis autoimmune disease |
|
|
nutritional imbalances |
vitamin deficiencies starvation overeating |
|
|
genetic |
sickle cell anemia red blood cell destruction |
|
|
possible responses of cell to injurious agents |
adaptation injury death |
|
|
adaptation |
cell undergoes changes that enable it to cope with excess stress - escapes injury |
|
|
injury |
reversible injury: if injurious agent removed, cell reverts back to normal state - morphologically and functionally irreversible injury: cell will not revert to normal, whether or not agent of injury is removed. Persistent or severe injury. Death of cell inevitable. |
|
|
death |
end stage of irreversible cellular injury necrosis or apoptosis |
|
|
response of cell to injury depends on |
- length of time of exposure to injurious agent: brief exposure may induce reversible, prolonged may cause irreversible - dose of injurious agent: small dose = minimal changes, large dose = cell death - type of cell and ability to adapt: neuron less able to adapt to hypoxia than cardiac muscle cell |
|
|
ischemia/hypoxia role of oxygen |
lack of oxygen within cell causes decrease of ATP production - biochemical mayhem |
|
|
role of oxygen in other injurious agents |
- capable of converting intracellular oxygen into oxygen-derived free radicals (reactive oxygen species) - superoxide (O2), hydrogen peroxide (h2O2), hydroxyl ion (OH) - very toxic - high concentrations destroy cellular proteins, membrane phospholipids, nucleic acid |
|
|
intracellular calcium and loss of calcium homeostasis |
- injurious agents can interfere with membrane-bound calcium ATP-ases (calcium pumps) - Ca+2 normally sequestered in organelles (mitochondria and ER) and outside cell enters cytosol - increase in cytosolic Ca+2 concentration - activation Ca+2 dependent enzymes injures cell |
|
|
Activated enzymes in Ca+2 loss of homeostasis |
- phospholipases: destroy membrane phospholipids - proteases: destroy proteins of cell membrane and cytoskeleton - endonucleases: DNA/chromatin fragmentation - ATPases: ATP depletion membrane damage, nuclear damage, depletion ATP |
|
|
increase in cytosolic Ca+2 effect on mitochondria |
- formation mitochondrial permeability transition pores within membrane - failure of oxidative phosphorylation - inability to generate ATP |
|
|
ATP depletion |
- activation of ATPases OR - decreased ATP synthesis - leads to loss of integrity of cell membrane |
|
|
defects in membrane permeability |
- direct damage by toxin OR - indirect through activation of Ca+2 dependent enzymes - involve plasma membrane as well as organellar membranes |
|
|
reversible time frame ischemic/hypoxic cell injury biochemical features |
- decrease/loss of ATP within cell due to decrease in oxidative phosphorylation by mitochondria - early biochemical events - if blood flow restored - cell will recover |
|
|
immediate consequences of loss of ATP within cell |
- leads to increase in anaerobic glycolysis (short term) - generate lactic acid - lowers intracellular pH - decreased activity/inactivation of intracellular enzymes - failure of sodium pumps on membranes - influx of sodium and water - swelling - impairs function |
|
|
Irreversible injury biochemical events |
- inability to reverse mitochondrial dysfunction even upon reoxygenation - ability to generate ATP permanently los - profound disturbances in plasma membrane function due to serious membrane damage |
|
|
profound plasma membrane damage in irreversible injury caused by |
- progressive loss of phospholipids from membrane - cytoskeletal abnormalities - reperfusion injury - lipid break down products - loss of intracellular amino acids results in cell death |
|
|
cause of progressive loss of phospholipids from membrane |
- phospholipid degradation by Ca+2 activated phospholipases - decrease in phospholipid synthesis due to lack of ATP |
|
|
cause of cytoskeletal abnormalities |
- Ca+2 proteases degrade cytoskeletal proteins - causes deformation of overlying membrane |
|
|
cause of reperfusion injury |
- blood flow and oxygen restored to irreversibly injured cells - large numbers of oxygen-derived free radicals may be generated - cause major membrane damage to injured cells - can also cause damage to healthy cells within area - extend area of tissue damage |
|
|
lipid breakdown products cause |
results from phospholipid degradation have detergent effect on membranes |
|
|
loss of intracellular amino acids cause |
- certain amino acids (glycine) protect membrane from hypoxic damage - loss leads to membrane injury |
|
|
Causes of cell death |
necrosis apoptosis autophagy |
|
|
cytoplasmic changes necrotic cells |
- increased eosinophilia: cytoplasm stains brilliant, deep pink when stained with hematoxylin and eosin (H and E)- due to move avid binding of eosin to denatured cytoplasmic proteins - vacuolization due to enzymatic digestion organelles - calcification |
|
|
nuclear changes necrotic cells |
Early on one of the following: - karyolysis - pyknosis - karyorrhexis 2 days - nucleus disappears |
|
|
karyolysis |
- nucleus stains very pale blue (vs. dark blue healthy) with H and E - due to DNA degradation by DNAses |
|
|
pyknosis |
- nucleus shrinks and becomes darker blue when stained with H and E - due to chromatin condensation |
|
|
karyorrhexis |
nucleus undergoes fragmentation |
|
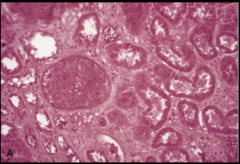
|
morphology of coagulative necrosis of tissue - outlines preserved and recognizable - occurs when denaturation of cellular proteins and enzymes predominates - cells do not autolyze - hypoxic/ischemic death |
|
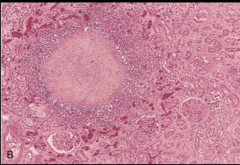
|
liquefactive necrosis of tissue - cells disappear due to digestion and lysis by enzymes - enzymes from either cell's lysosomes or white blood cells in area (heterolysis) - characteristic of focal bacterial infections |
|
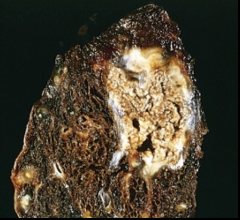
|
Caseous necrosis of necrotic tissue - appears cheesy - necrotic focus of fragmented cells and granular cellular debris surrounded by granulomatous inflammation - characteristic of tuberculosis (TB) |
|
|
apoptosis |
- distinctive pattern of cell death - induced by activation of intracellular suicide program - activation of intraceullar enzymes that degrade nucleus and cytoplasmic proteins - specific physiologic and pathologic circumstances |
|
|
physiologic instances of apoptosis |
- embryogenesis and development - immature cells routinely die and replaced by mature ones - hormonal dependent involution in adult tissues (menstruation) |
|
|
pathologic examples apoptosis |
- cell death in tumors (tumor necrosis factor) - cell death in certain viral diseases (hepititis) - others |
|
|
initiation phase apoptosis - intrinsic (mitochondrial) pathway |
1) stressor activates intracytoplasmic sensor proteins 2) sensors of subfamily Bcl-2 proteins called BH3- only (Bim, Bid and Bad) 3) activate Bax and Bak (pro-apoptotic) - polymerize into oligomers and insert on mitochondrial membrane, creates channels to allow proteins out - also inhibits anti- apoptotic Bcl-x and Bcl-2 4) release mitochondrial pro-apoptotic proteins (CYTOCHROME C) into cytoplasm 5) cytochrome C bind apoptosis-activating factor -1 (Apag-1) - forms hexamer apoptosome 6) apoptosome activates critical initiator caspase (caspase-9) - activates adjacent molecules - caspase cascade |
|
|
extrinsic (death receptor) pathway - initiation phase apoptosis |
- death receptors: deliver apoptotic signals (type 1 TNF receptor - TNFR1 and Fas) 1) ligand bind to death receptor 2) activation initiator caspases 8 and 10 3) activation other initiator caspases 4) initiator caspase cascade |
|
|
execution phase |
1) activated initiator caspases activate executioner caspases (3 and 6) 2) degradation critical cellular components (DNA, nuclear proteins, etc) 3) cell death |
|
|
morphologic characteristics apoptosis |
- single cells or small groups - minimal membrane damage - nuclear chromatin condenses and aggregates peripherally against nuclear membrane - pronounced blebbing (outpouchings of cell membrane due to decoupling cytoskeleton) - membrane bound apoptotic bodies - phagocytosed by macrophages/phagocytic cells - no inflammatory response - rapid phagocytosis |
|
|
autophagy phsiology |
- provides survival mechanism for nutritionally deprived cells - autophagic vacuole - contains organelles - fuses with lysosome - enzymatic digestion organelles, release organic materials to cytoplasm - nutrients for cell survival |
|
|
autophagy pathology |
- run away, up-regulated physiology pathway - too many organelles destroyed = cell death - several disease processes: degenerative diseases nerve and muscle (alzheimer's, ALS) |
|
|
autophagy genetics and cell death |
- process regulated by Atg genes - unclear whether direct cause of cell death or results from stressor - role in defense of viral and bacterial infections |
|
|
Necrosis vs apoptosis |

|

