![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
How is cholesterol stored inside cells? What is the enzyme involved? (p240)
|
It is esterified by acyl-CoA-cholesterol acyl transferase (ACAT).
|
|
|
What form of cholesterol is found in membranes and what form is found in storage?
|
The sterol with a hydroxyl group is found in membranes. The sterol with an ester group replacing the hydroxyl group is the stored form (as droplets inside cells)
|
|
|
In what form is cholesterol typically found floating around in the serum?
|
In the esterified form.
|
|
|
To minimize the risk of cardiovascular events, what levels of LDL and HDL are idea? (p241)
|
Low LDL and high HDL is best.
L=LDL H=HDL |
|
|
Cholesterol on its own is not very soluble, however in the serum, concentrations are very high. How is this possible?
|
Due to its association with HDL and LDLs.
|
|
|
All 27 carbons of cholesterol are derived from what? (p242)
|
Acetate. Specifically, Acetyl-CoA
|
|
|
There are 37 steps in the biosynthesis of cholesterol however we're breaking it down into four stages. What are those stages?
|
1. Acetate (3C) is converted to mevalonate (6C).
2. Mevalonate is converted to an activated isoprene (5C with double bond) 3. Activated isoprene is condensed to 30C molecule called Squalene. 4. Squalene is converted to cholesterol |
|
|
Although cholesterol can be synthesized in all cells, where is it most readily produced? Why? What are three others? Why? (p242, my written note in blue)
|
In the liver due to bile synthesis. Other tissues include intestine, adrenal cortex, and reproductive tissues. These tissues manufacture a lot of cholesterol for hormone purposes.
|
|
|
Stage 1: Formation of Mevalonate
1. Where does this rxn occur? 2. What three sources can Acetyl-CoA be derived? (p243 top) |
1. In cytosol
2. B-oxidation of FA, oxidation of ketogenic aa (e.g. leucine, isoleucine), pyruvate dehydrogenase rxn. |
|
|
1. What is the enzyme of the major regulatory point in cholesterol synth?
2. Where is it located? 3. What energy source does it use? 4. What rxn does it catalyze? |
1. HMG-CoA reductase
2. Integral protein of ER with active site exposed to cytosol. 3. Requires NADPH for E 4. Catalyzes hydrolysis of thioester bond, generating an alcohol group. |
|
|
There are two isoforms of HMG-CoA SYNTHASE - where are they located and what are they involved in? (p243)
|
1. Cytosol isoform is involved in cholesterol synthesis.
2. Mitochondrial isoform is involved in ketone body synthesis. **these rxns are identical - only in different locations** |
|
|
What is both the committed and rate limiting step in cholesterol synthesis?
|
HMG-CoA REDUCTASE
|
|
|
The first two steps in cholesterol synth are identical to:
|
Ketone body synthesis in the mitochondria.
|
|
|
2 Acetyl-CoA is condensed to ? by this enzyme:
|
Acetoacetyl-CoA via thiolase
|
|
|
Acetoacetyl-CoA combines with an Acetyl-CoA to form ? by this enzyme:
|
beta-Hydroxy-beta-Methylglutaryl-CoA (HMG-CoA) via HMG-CoA synthase
|
|
|
HMG-CoA is reduced to ? by this enzyme that requires these energy co-factors:
|
Mevalonate via HMG-CoA reductase; requires 2 NADPH molecules.
|
|
|
Mevalonate is converted to ? via this enzyme, requiring what energy?
|
5-Phosphomevalonate via Mevalonate-5-phosphotransferase requiring 1 ATP
|
|
|
5-Phosphomevalonate is converted to ? via this enzyme that requires this energy unit?
|
5-pyrophosphomevalonate via Phosphomelavonate Kinase, requiring 1 ATP
|
|
|
5-pyrophosphomevalonate is converted to this ? intermediate via this enzyme - that also decarboxylates the intermediate for form what?
|
5-pyrophosphomevalonate is phosphorylated to 3-Phospho-5-Pyrophosphomevalonate [intermediate] via pyrophosphomevalonate decarboxylase - this is decarboxylated (giving off CO2) by the same enzyme to produce activated isoprenes: delta3-Isopentenyl pyrophosphate and Dimethylallyl pyrophsosphate.
|
|
|
The only difference between delta 3-isopentenyl pyrophosphate and Dimethylallyl pyrophosphate is:
|
The position of the double bond.
|
|
|
Two activated isoprenes are condensed head to tail via this enzyme to make a 10C molecule called? Note that once this happens, another activated isoprene is added using the same enzyme to produce a 15-carbon molecule called?
|
Prenyl Transferase makes a 10C compound called Geranyl Pyrophosphate. The same enzyme adds another activated isoprene to make a 15C compound called Farnesyl Pyrophosphate.
|
|
|
Two molecules of Farnesyl Pyrophosphate are combined via this enzyme that requires this energy subunit to produce a 30 Carbon molecule called what?
|
They combine via squalene synthase, requiring NADPH, and produce a molecule called Squalene.
|
|
|
What does squalene monooxygenase do? (p246)
|
It converts squalene into an epoxide.
|
|
|
Once squalene is made, it is ready to be converted to a four-ring steroid nucleus. 1st Squalene must be converted to a ? with this enzyme that requires this energy unit: (p246)
|
Squalene is converted to an epoxide - Squalene 2,3-epoxide - via Squalene Monoxygenase requiring 1 NADPH and OXYGEN
|
|
|
Once the epoxide, Squalene 2,3-epoxide is made, it is cyclized to ? via this enzyme?
|
Converted to Oxidosqualene via Oxidosqualene Cyclase to form Lanosterol.
|
|
|
Once Lanosterol is produced, there is a multistep process to finally make cholesterol. What are the three major events during this multistep process?
|
1. Dimethylation
2. Double bond reduction 3. Double bond movement. |
|
|
It was confusing at first how cholesterol pathway disorders could affect development until researchers realized:
|
that cholesterol can be attached to proteins and affect their activity. Example include hedgehog family of transcription factors.
|
|
|
What are SREBPs and how do they function?
|
They are Sterol Regulatory Element-Binding Proteins. When cholesterol is high, SREBPs are inactive and retained in the ER. When cholesterol levels decline, SREBP undergoes proteolytic cleavage, releasing a region of the protein that enters the nucleus where it acts as a transcription factor by binding to sterol reg elements (SRE) in the DNA to activate transcription of sterol-regulated genes.
|
|
|
See p247 for pathway that results in SREBP cleavage to make TF that turns on sterol synthesis.
|
See p247 for pathway that results in SREBP cleavage to make TF that turns on sterol synthesis.
|
|
|
How is HMG-CoA reductase regulated in terms of its phosphorylation?
|
The active form is dephosphorylated (insulin) and the inactive form is phosphorylated (glucagon)
|
|
|
After a meal, is HMG-CoA reductase turned on or off?
|
After a meal, HMG-CoA is dephosphorylated by insulin and therefore activ.
|
|
|
What are the three major ways in which HMG-CoA reductase can be regulated?
|
1. Transcriptional
2. Phosphorylation 3. Proteolysis/degradation. |
|
|
How do statins work?
|
They act as competitive inhibitors of HMG-CoA reductase.
|
|
|
What was the pleiotropic action of statin example she used in class?
|
Where blocking HMG-CoA reductase, you also block modification of geranylgeranyl that increases the expression of eNOS - and therefor improve endothelial function.
|
|
|
Bile acids are derivatives of ?, a #-carbon molecule with # hydroxyl groups.
|
Derivative of cholic acid, a 24-carbon molecule with 3 hydroxyl groups.
|
|
|
What three important things does bile acids do?
|
1) They act as emulsifying agents to prepare dietary triacyglycerols for hydrolysis by pancreatic lipase. 2) They facilitate the absorption of fat-soluble vitamins, esp Vit D. 3) Since cholesterol is not oxidized to CO2 and H2O, it is excreted as free cholesterol and as bile acids.
|
|
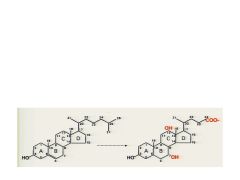
Name these structures. What is the one on the right useful for and how is it different than the one on the left.?
|
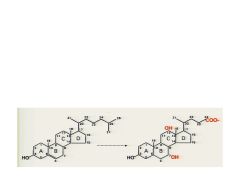
Cholesterol and Cholic Acid. It is useful form of cholesterol that can be excreted. It has additional OH groups, fewer Carbons, and is more water soluble.
|

