![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
179 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Blank
|
Blank
|
|
|
|
What are the three major components of the nucleolus?
|
Three major components of the nucleolus are recognized: the fibrillar centers (FC), the dense fibrillar component (DFC) and granular components (GC).
|
|
|
|
What 3 factors make up platelet granulocytes?
|
Granules: lysosomes
a. alpha: fibrinogen, platelet derived growth factor, [PDGF]etc. b. delta: calcium, serotonin, ATP, etc. c. lambda: lysosomal(hydrolytic) enzymes Serotonin is a potent vasoconstrictor. The release of serotonin from thrombocytes, which adhere to the walls of a damaged vessels, is sufficient to close even small arteries. |
|
|
|
Describe the morphological differences between ulceration and erosion
|
An ulcer is an erosion of more than 0.5 cm.
|
|
|
|
Describe the function of plasma cells, lymphcytes, monocytes/macrophages, fibroblasts and eosinophils
|
Plasma cells are differentiated B lymphocytes that produce antibodies.
Lymphocytes include both helper and cytotoxic T cells as well as B cells. Macrophages (Ma) are phagocytic and antigen-presenting. Eosinophils (E) release major basic protein - effective in killing parasites. Fibroblasts secrete extracellular matrix including collagen. After the injurious stimulus has been removed these cells progressively disappear from the tissue over weeks/months. |
|
|
|
Name the bacterial infection usually associated with chronic ulceration of the stomach and duodenum
|
Helicobacter pylori, a spiral-shaped bacterium that lives in the acidic environment of the stomach
|
|
|
|
Define the terms "Hematemesis" and "Melena"
|
Hematemesis: The vomiting of blood. The source is generally the upper gastrointestinal tract.
Melena: The black, "tarry" feces that are associated with gastrointestinal hemorrhage. The black color is caused by oxidation of the iron in hemoglobin during its passage through the ileum and colon. |
|
|
|
Describe the typical outcome of chronic inflammation
|
Tissue destruction, fibrosis, necrosis
|
|
|
|
Describe the definitive features of a granuloma
|
Activated epithelioid macrophages and multinucleate giant cells derived from macrophages
Epithelioid macrophages form circumscribed granulomas (clusters) surrounded by lymphocytes, macrophages, fibroblasts and varying degrees of fibrosis |
|
|
|
Describe the formation of epithelioid macrophages and giant cells
|
Formed in response to a persistent irritant where several macrophages, along with other WBCs, fuse into a larger cell, sometimes appearing like epithelium. Their purpose is to isolate the irritant.
|
|
|
|
Compare and contrast granulation tissue vs. granuloma
|
Granulation tissue is an intermediate stage of acute healing which is later replaced by collagen.
A granuloma is a permanent answer to a persistent irritant. |
|
|
|
Compare and contrast foreign body giant cells and Langhan's giant cells
|
A foreign-body giant cell is a collection of fused macrophages (giant cell) which are generated in response to the presence of a large foreign body. This is particularly evident with implants that cause the body chronic inflammation and foreign body response.
The nuclei are arranged in a disorganized manner. This is in contrast to a Langhans giant cell, where the nuclei are arranged on the border. |
|
|
|
Predict cellular activity based on the chromatin structure
|
Condensed - Interphase
Extended - Immediately before M Phase Chromatin concentration, formation of kinetochores - Prophase Chromatids at center - Metaphase Sister Chromatids seperated - Anaphase Sister Chromatids at opposite poles - Telophase Two joined daughter cells - Cytokinesis |
|
|
|
List and describe the sequence of events occuring in interphase
|
S phase
– Replication of DNA – Replication of centrosome G2-to-M transition – two centrosomes separate – move to opposite poles – orientation determines the |
|
|
|
List two stains for staining elastic fibers
|
Orcein, resorcin or Verhoeff's
|
|
|
|
Identify the cell types responsible for the production of ground substance
|
mesenchymal cells (stem cells)
|
|
|
|
Describe the glands of the gastric mucosa - cardiac glands.
|
Secretion: along with that of esophageal cardiac glands contributes to gastric juice
•tubular, tortuous, sometimes branched glands, composed of mucous secreting cells with a short duct segment •connects gland with shallow gastric pits •some enteroendocrinecells interspersed |
|
|
|
Describe the basic chemical composition of ground substance
|
primarily composed of glycosaminoglycans (most notably hyaluronan), proteoglycans, and glycoproteins and water
|
|
|
|
Describe the structure and function of the centromere
|
Centromere––Direct movement of chromosome ––Centric ––Persists throughout interphaseinterphase••
|
|
|
|
Describe the structure and function of the kinetochore
|
Kinetochore––Large protein complex (>80 proteins)––Forms near the centromere––Attach chromosome to mitotic spindle
|
|
|
|
Name and describe the glands of the gastric mucosa - parietal oxyntic cells
|
OXYNTIC CELLS secrete HCl and intrinsic factor
Located in neck among mucous neck cells •Large, often binucleate, cells give glandular epithelium beaded appearance. •Appear triangular with apex directed toward lumen of gland •EM shows extensive INTRACELLULAR CANALICULAR SYSTEM which communicates with lumen of fundicgland. Numerous surface microvilliproject from canaliculi where the HCl is produced. |
|
|
|
Name and describe the glands of the gastric mucosa - chief cells
|
•Typical protein-secreting cells
•Loactedin the deepest part of fundicglands •Cuboidalor low columnar •Cells easily identified by intense basophilia caused by basal RER and apical granules •Secrete: pepsinogen (a weak lipase) |
|
|
|
Name and describe the glands of the gastric mucosa - enteroendocrines.
|
Small cells located at any level of gland sitting on the basal lamina.
•Cells hard to identify but clear cytoplasm stands out in contrast to chief cells •Produce gastrin, secretin,cholecystokinin into lamina propria. |
|
|
|
Name the structure and function of villus.
|
•Epithelium--simple columnar
•Finger-like; leaf-like projections •Core of villus consists of 1) extension of lamina propria containing a blind ended central lacteal accompanied by smooth muscle. 2) network of fenestrated capillaries beneath |
|
|
|
What are the Valves of Kercking? What do they do?
|
PLICAE CIRCULARES: permanent transverse folds that contain a core of submucosa. They slow the passage of the food along the intestines, and afford an increased surface for absorption. They are covered with villi which absorb fats and nutrients from the chyme.
|
|
|
|
Cilia and Chronic Sinusitis, Recurrent Ear Infections and Bronchiectasis
|
Cilia damage or mutation is common from Chronic Sinusitis. ciliated cells are lost and replaced by columnar epithelium with villi.
|
|
|
|
Kartagener's Syndrome
|
Immotile ciliary syndrome. A defect in the action of the cilia lining the respiratory tract
Poor mucus clearance causes chronic infection. Also responsible for situs inversus and sterility in males. |
|
|
|
Polycystic Kidney Disease
|
Large, fluid-filled cysts enlarging the kidneys. The disease can also damage the liver, pancreas, and in some rare cases, the heart and brain.
|
|
|
|
Bullous Vesicular Lesions
|
An acute or chronic autoimmune skin disease, involving the formation of bullae (blisters) between epidermis and dermis. The bullae are formed by an immune reaction as autoantibodies target hemidesmosomes. Results in a separation along the dermal-epidermal junction and eventually stretch bullae.
|
|
|
|
Epithelial Carcinoma
|
Tumors can arise from epithelial tissue - carcinoma.
Benign: Chondroma, Lipoma Malignant: Carcinoma, Sarcoma |
|
|
|
Epithelial Metaplasia
|
When cells are faced with physiological or pathological stresses they respond by adapting in several ways, possibly changing into another, more protected type of epithelium.
|
|
|
|
Histamine and Heperin
|
Vasodilators
|
|
|
|
Describe the duodenum.
|
•Duodenum
- plicaecircularis - Long prominent villi - fewer goblet cells - Brunner's Glands |
|
|
|
Describe the progressive changes of the Jejunum
|
•Jejunum
-Long prominent villi -More goblet cells -No submucosalglands |
|
|
|
Describe the progressive changes seen in the ileum.
|
•Ileum
-Short villi -Goblet cells increase -Peyer’s patches(GALT) -Epithelium covering peyerspatches –M cells |
|
|
|
Describe the tunics of the large intestine - mucosa
|
Mucosa
•Contains numerous CRYPTS OF LIEBERKUHN •Simple columnar epithelium –absorb water and electrolytes •Goblet Cells •No Paneth cells(except appendix) |
|
|
|
Predict cellular activity based on the chromatin structure
|
Condensed - Interphase
Extended - Immediately before M Phase Chromatin concentration, formation of kinetochores - Prophase Chromatids at center - Metaphase Sister Chromatids seperated - Anaphase Sister Chromatids at opposite poles - Telophase Two joined daughter cells - Cytokinesis |
|
|
|
Describe the role of cyclins and cyclin-dependent kinases in cell cycle regulation.
|

A family of proteins that control the progression of cells through the cell cycle by activating cyclin-dependent kinase (Cdk) enzymes by cyclically varying their concentration.
Upon phosphorylation form maturation-promoting factor (MPF). MPFs activate other proteins through phosphorylation and these other proteins are responsible for specific events such as microtubule formation and chromatin remodeling. |
|
|
|
List and describe the checkpoints of cell cycle regulation.
|
G1 Checkpoint (Restriction Point) –end of G1 phase, Decides whether the cell should divide or enter G0.
G2 Checkpoint – end of G2, triggers M Phase if the cell’s DNA is not damaged and the cell is ready for mitosis. Metaphase Checkpoint (Spindle Checkpoint) – occurs in metaphase when all chromosomes should have aligned at the mitotic plate, beginning Anaphase. |
|
|
|
Reticular CT
|
1. Reticular cells (modified fibroblasts) -cytoplasmicextensions cover reticular fibers
2. Framework for myeloid (bone marrow) and lymphoid (lymph nodes, spleen) organs |
|
|
|
Describe the changes in the autonomic nerve plexus in the wall of the GIT that may lead to Hirschsprung Disease (congenital mega colon).
|
Congenital disorder of the colon where ganglion cells in the myenteric plexus, responsible for moving food, are absent.
Symptom - chronic constipation Treatment - "pull-through" surgery. |
|
|
|
What is GERD? Barrett's Esophogus?
|
Gastroesophageal Reflux Disease (GERD)
Symptoms - Acid Reflux and Heartburn Prognosis - Metaplasia with eventual dysplasia as epithelium changes to columnar. Treatment - Medicine while mild, Surgery where chronic (Fundiplication) Barrett's: May lead to invasive adenocarcinoma of the esophagus (with poor prognosis) |
|
|
|
Explain the consequences of varicosities of the esophagus and the rectum.
|
Esophagus - linked with liver cirrhosis
Rectum - referred to as piles or hemorrhoids Prognosis - danger of blood clots, deep vein inflammation or thrombophlebitis leading to pulmonary embolism and possible death. Women are twice as likely to develop varicose veins than men. |
|
|
|
Describe the changes that occur in the wall of the gastro intestinal tract after surgical procedures like appedectomy, which may lead to adhesions.
|
Abnormal connections to abdominal organs by thin fibrous (scar) tissue
Cause - a complication of abdominal surgery Prognosis - intestinal obstruction preventing normal flow of intestinal contents, possible hernia |
|
|
|
Describe the changes in the villi in malabsorption syndrome.
|
Coeliac sprue - autoimmune inflammatory disorder caused by intolerance to gluten
Prognosis - inflammation of the intestines, vitamin deficiencies due to lack of absorption of nutrients, and bowel abnormalities. |
|
|
|
Describe the changes to the wall of the stomach in the development of ulcers. Aslo describe the effect of aspirin (NSAIDs) on the wall of stomach. Describe the main complications of chronic peptic ulceration.
|
Peptic Ulcer Disease - an open sore that develops on the inside lining of the stomach (gastric ulcer), or the small intestine (duodenal ulcer). Gastrointestinal bleeding when the ulcer erodes a blood vessel. Blood is a laxative and the loss of fluids can become life threatening.
Perforation at the anterior surface of the stomach leads to acute peritonitis. Perforation of the gastro-intestinal wall may lead to spillage of stomach or intestinal content into the abdominal cavity with possibly catastrophic consequences. Cause - inflamation of the epithelial lining - caused by Helicobacter pylori or use of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and ibuprofen. |
|
|
|
Describe the histological appearance of a adenomatous polyp of the large intestine.
|
Abnormal overgrowth of tissues protruding from the mucous membrane
Prognosis - higher risk of colon cancer though most are benign. Treatment - surgery Three types - villous, tubular and tubulovillous |
|
|
|
Define the term “sialolith”
|
Stone in the salivary ducts (usually submaxillary) or glands.
|
|
|
|
Describe the main features of pleomorphic adenoma
|
Benign neoplastic tumor of the salivary glands consisting of mixed epithelial and mesenchymal cell components.
|
|
|
|
Define the term “gastric dysplasia”
|
Neoplastic epithelium, considered both a carcinoma precursor and a marker of high cancer risk for the site at which it is found. Symptoms - atypical cells, abnormal differentiation or disorganized mucosal architecture
|
|
|
|
List the main features of gastrointestinal carcinoid tumors
|
Cause - Endogenous secretion of serotonin and kallikrein in crypts of Lieberkühn.
Symptoms - flushing, diarrhea, cardiac abnormalities with possible brochioconstriction or even heart failure. |
|
|
|
what is chronic inflammatory bowel disease and list the two major types.
|
Crohn’s disease, or regional enteritis, is an inflammatory disease where the ulcerated stage (mucosal loss and denudation) is associated with intense inflammation and later fibrosis and contracture.
Ulcerative Colitis - acute inflammation of the mucosa with neutrophils accumulating in the lamina propia and in the lumina of the colonic glands to form crypt abscesses. Depletion of goblet cells and ulceration of the mucosa also occur. Cause - a distortion of crypt architecture, inflammation of crypts (cryptitis), frank crypt abscesses, and hemorrhage or inflammatory cells in the lamina propria. |
|
|
|
What is diverticular disease.
|
Diverticula can pass through the muscular layer of the bowel, classically, the sigmoid colon.
Acute: obstruction of fecal matter with infection, obstruction, bleeding and perforation. Infection may lead to the formation of an abscess or even a fistula |
|
|
|
List the main features of adenocarcinoma of the colon
|
A malignant tumor in glandular epithelium. Due to polyps. The bigger the adenoma, the more likely it is to become cancerous.
|
|
|
|
Describe pseudomembranous colitis.
|
An infection of the large intestine from the toxin of Clostridium difficile. Normally present in the intestine, it may overgrow with antibiotics.
Symptoms - the lining of the colon becomes inflamed and bleeds, and takes on a characteristic appearance called pseudomembranes. |
|
|
|
what is Candida albicans
|
Candida albicans, a fungus normally living in the mouth and the gastrointestinal tract, overgrows when the immune system is weakened. Common locations of infection are skin, mouth, genital areas, urinary tract.
|
|
|
|
Describe the role of the anaphase-promoting complex in mitosis and cell cycle regulation
|
Once the Metaphase Checkpoint is passed (chromosmes are at mitotic plate under bipolar tension) cyclin B is degraded so that it no longer inhibits the anaphase-promoting complex, which is now free to break down securin which is inhibiting separase, the protein responsible for the separation of the sister chromatids.
|
|
|
|
List and describe the sequence of events occuring in meiosis.
|
Meiosis I – separation of chromosomes from 2n - 1n Prophase I – Nuclear envelope disappears. Exchange of DNA with chromosomal crossover of chiasmata.
Metaphase I – Homologous pairs move to metaphase plate, microtubules attach at kinetochores. Anaphase I – Microtubules shorten, separating the chromosomes. Telophase I – Ends when chromosomes reach poles. Cytokinesis then occurs, creating two attached daughter cells. Interphase (Interkinesis) II Meiosis II – double the number of 1n cells Prophase II – Nuclear envelope disappears again. Chromatids again shorten and thicken. Centrioles move to poles. Metaphase II – Chromosomes aligned on a new metaphase plate, perpendicular to the first. Anaphase II – Centromeres are cleaved, microtubules to pull chromatids apart. Telophase II – uncoiling of the chromosomes and disappearance of the spindle. Nuclear envelope reforms producing four daughter cells. |
|
|
|
List and describe the 5 stages of of Prophase I in meiosis.
|
Leptotene – Homologous chromosomes condense & connect to each other. Homo
Zygotene - Pairing of homologous chromosomes (Synapsis) with formation of a Synaptnemal Complex binding chromosomes together Pachytene - Completion of Synapsis and crossing over Diakinesis - Homologous chromosomes condense as Nucleolus disappears and nuclear envelope disintegrates Diplotene - Synaptonemal complex breaks down, homologous chromosomes separate |
|
|
|
List and describe the sequence of events occuring in interphase
|
S phase
– Replication of DNA – Replication of centrosome G2-to-M transition – two centrosomes separate – move to opposite poles – orientation determines the |
|
|
|
Describe the intrinsic and extrinsic apoptosis pathways
|
Extrinsic - Ligand binds to death receptor, recruiting death domain adaptor proteins. Activation of initiator then effector caspases
Intrinsic Death signal (e.g. Oxidative stress) releases Cytochrome c from the mitochondria with formation of apoptosome and activation of caspase 9 and effector caspases |
|
|
|
Describe the classification of cancer cells including carcinoma, sarcoma and leukemia
|
Carcinoma - an invasive malignant tumor consisting of transformed epithelial cells.
Sarcoma - a cancer that arises from transformed connective tissue cells. Leukemia - a type of cancer of the blood or bone marrow characterized by an abnormal increase of white blood cells. |
|
|
|
Define oncogene and tumor suppressor gene
|
Oncogene - a gene that has the potential to cause cancer.
Tumor Suppressor Gene - an anti-oncogene, a gene that protects a cell from one step on the path to cancer. |
|
|
|
Describe the structure and function of the kinetochore
|
Kinetochore––Large protein complex (>80 proteins)––Forms near the centromere––Attach chromosome to mitotic spindle
|
|
|
|
Describe the role of the retinoblastoma (Rb) and p53 tumor suppressor genes in the cell cycle and cancer
|
The retinoblastoma protein (pRB or Rb) is a tumor suppressor protein that regulates the cell cycle. Should an oncogenic protein bind and inactivate pRb, this can lead to cancer.
p53 is a tumor suppressor that is involved in preventing cancer. |
|
|
|
Define and compare/contrast histopathology versus cytopathology
|
Cytopathology - Cytopathology - a branch of pathology that studies and diagnoses diseases on the cellular level.
Histopathology - the microscopic examination of tissue in order to study the manifestations of disease. |
|
|
|
Describe the principles of Erythropoiesis
|
Pleuripotent cells - Multipotent cells - Pro___blasts - _____blasts - _______cytes - Meta_____cytes - Mega_____cytes.
|
|
|
|
Define cellular degeneration and list 3 common structural changes
|
Cellular degeneration is a result of sub-lethal damage.
Changes include: mitochondrial swellings: at first, spaces of vacuoles develop within the mitochondrion, distorting the cristae; eventually the cristae will be destroyed, which will permanently damage the mitochondrioin swelling of ER and loss of ribosomes attached to RER cell appears to swell and beocome pale (cloudy swelling and hydropic degeneration) |
|
|
|
Describe the histological appearance of cloudy swelling and hydropic degeneration, and explain the cause of these changes
|
Microscopically, these reversible changes caused by swelling of the organelles is reflected in cellular swelling, paleness of cytoplasm and development of small intracellular vacuoles, giving rise to the widely used descriptive terms cloudy swelling and hydropic degeneration.
|
|
|
|
Describe the histological appearance of fatty degeneration. List the types of cells in which it occurs and explain the cause of this change.
|
the process describing the abnormal retention of lipids within a cell. It reflects an impairment of the normal processes of synthesis and elimination of triglyceride fat. Excess lipid accumulates in vesicles that displace the cytoplasm.
|
|
|
|
Describe the changes of antigens on the surface of red blood corpuscles that may lead to Rh incompatibility and erythroblastosis fetalis
|
A mother is exposed to a foreign antigen and produces IgG, which will target the antigen, if present in the fetus, attacking its erythrocytes and causing anemia.
If a mother has anti-RhD (D being the major Rhesus antigen) IgG antibodies as a result of previously carrying a RhD-positive fetus, this antibody will only affect a fetus with RhD-positive blood. |
|
|
|
Name the mitochondrial enzyme which is released into cytoplasm in apoptosis from many different causes.
|
SMACs (second mitochondria-derived activator of caspases) are released into the cytosol following an increase in permeability. SMAC binds to inhibitor of apoptosis proteins (IAPs) and deactivates them, preventing the IAPs from arresting the apoptotic process and therefore allowing apoptosis to proceed.
|
|
|
|
Describe the enzyme sysgtem which is the final effector mechanism for apoptosis.
|
Several proteins are involved, but two main methods of regulation have been identified: targeting mitochondria functionality, or directly transducing the signal via adaptor proteins to the apoptotic mechanisms. Another extrinsic pathway for initiation identified in several toxin studies is an increase in calcium concentration within a cell caused by drug activity, which also can cause apoptosis via a calcium binding protease calpain.
|
|
|
|
List 5 common injurious stimuli whcih can trigger apoptosis
|
- ligation of cell surface receptors
- DNA damage as a cause of defects in DNA repair mechanisms - treatment with cytotoxic drugs or irradiation - lack of survival signals, contradictory cell cycle signalling - developmental death signals. |
|
|
|
List examples of physiological processes involving apoptosis
|
Any loss of macro structures such as webbed fingers, tails, etc. Children lose something like 30 billion cells a day to apoptosis.
|
|
|
|
Describe the process of autodigestion or autolysis of the cell and the oganelle primarily responsible.
|
autolysis, more commonly known as self-digestion, refers to the destruction of a cell through the action of its own enzymes. It may also refer to the digestion of an enzyme by another molecule of the same enzyme.
|
|
|
|
Define and compare/contrast pyknosis, karyorrhexis and karyolysis
|
Pyknosis (Karypyknosis) - irreversible condensation of chromatic in the nucleus prepatory to apoptosis or necrosis.
Karyorrhexis - Fragmentation of the nucleus before cell death Karyolysis - Complete dissolution of the chromatin matter of a dying cell due to the activity of DNase. Occurs mainly as a result of necrosis, while in apoptosis after karyorrhexis the nucleus usually dissolves into apoptotic bodies. |
|
|
|
Describe the structural changes that occur in cells after apoptosis is triggered
|
These changes include blebbing, loss of cell membrane asymmetry and attachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation.
|
|
|
|
Describe the appearance of apoptotic bodies
|
Small, sealed membrane vesicles which dye uniformly.
|
|
|
|
List different clinical conditions where neutrophils, eosinophils and basophils are elevated in peripheral smear, respectively.
|
Neutrophil - bacterial infection
Eosinophil - parasitic infection Basophil - alergic reaction (histamine) |
|
|
|
Interpret the changes that constitute Pancytopenia (Bone Marrow Failure)
|
anemia: hemoglobin < 13.5 g/dL (male) or 12 g/dL (female)
neutropenia: Absolute Neutrophil Count (ANC) < 1.5×103/microliter thrombocytopenia: platelet count < 150×109/L |
|
|
|
Describe the changes involved in the hemoglobin molecule that results in thalassemia
|
A genetic defect, which could be either mutation or deletion, results in reduced rate of synthesis or no synthesis of one of the globin chains that make up hemoglobin. This can cause the formation of abnormal hemoglobin molecules, thus causing anemia, the characteristic presenting symptom of the thalassemias.
|
|
|
|
List a minimum of four causes for anemia.
|
Impaired production - disturbed stem cells. Aplastic anemia, renal failure, endocrine disorders, disturbance of erythroblasts, Pernicious anemia (Vitamin B12 deficiency), folic acid deficiency. Iron deficiency anemia, Thalassemias, Myelophthisic anemia or (replacement of bone marrow by tumors or granulomas), chronic inflammation
Increased destruction - Hemolytic anemia Enzyme deficiencies - Pyruvate kinase and hexokinase deficiencies, Glucose-6-phosphate dehydrogenase deficiency and glutathione synthetase deficiency, causing increased oxidative stress Hemoglobinopathies - Sickle cell anemia, Rh disease, transfusion reaction,Mechanical trauma to red cells, Infections, including malaria Blood loss - Trauma or surgery, gastrointestinal tract lesions, gynecologic disturbances, |
|
|
|
List the basic classification of leukemia and the major difference between them
|
Acute - a rapid increase in the numbers of immature blood cells. Crowding makes the bone marrow unable to produce healthy blood cells.
Chronic - excessive build up of abnormal white blood cells. lymphoblastic or lymphocytic leukemias: cancer in the precursor to B lymphocytes. myeloid or myelogenous leukemias: cancer in a precursor to other WBCs, RBCs or platelets |
|
|
|
Define the term "polycythemia vera"
|
erythremia - a blood disorder in which the bone marrow makes too many red blood cells and may also result in the overproduction of white blood cells and platelets leading to thickening
|
|
|
|
Define essential thrombocytemia
|
A rare chronic blood disorder characterized by the overproduction of platelets by megakaryocytes in the bone marrow.
|
|
|
|
Define Lipoma
|
Benign tumor composed of adipose tissue
|
|
|
|
Define Liposarcoma
|
A malignant tumor that arises in fat cells in deep soft tissue, such as that inside the thigh or in the retroperitoneum.
|
|
|
|
Where do macrophages come from and how long do macrophages live?
|
Macrophages originate from monocytes, which migrate to connective tissue and differentiate into tissue macrophages. Macrophages are normally l
In some cases where a foreign body (such as a small splinter) has penetrated the inner tissues of the body, several macrophages may fuse together to form multinuclear foreign body giant cells. These large cells accumulate at sites of invasion of the foreign body and sites of inflammation. |
|
|
|
How long do Plasma Cells live? Where do they live?
|
Plasma cells are relatively short-lived (10-20 days) and are found in sites of chronic inflammation or sites of high risk of invasion by bacteria or foreign proteins.
|
|
|
|
Leukocytes
|
The white blood corpuscles are commonly found in connective tissue. They migrate from the blood vessels to the connective tissue, especially to sites of injury or inflammation.
|
|
|
|
Describe the process of photoaging
|
Both UVA and UVB radiation can cause skin damage including wrinkles, lowered immunity against infection, aging skin disorders, and cancer. Some of the possible mechanisms for UV skin damage are collagen breakdown, the formation of free radicals, interfering with DNA repair, and inhibiting the immune system.
|
|
|
|
Describe the role of myofibroblasts in wound repair and inflammation.
|
Wound contraction - actin fillaments contract when exposed to substances that cause smooth muscle to contract, such as adrenaline or angiotensin.
Transform into myofibroblasts After healing is complete, these cells are lost through apoptosis |
|
|
|
Tumor Necrosis Factor
|
A cytokine involved in systemic inflammation and the regulation of immune cells. TNF is able to induce apoptotic cell death, to induce inflammation, and to inhibit tumorigenesis and viral replication.
Dysregulation of TNF production may cause major depression, Alzheimer's disease and cancer. |
|
|
|
List and describe the three major components of the acute inflammatory response
|
Vascular dilatation - relaxation of vascular smooth muscle leads to engorgement of tissue with blood (hyperemia)
Endothelial activation - increased endothelial permeability allows plasma proteins to “leak” into tissues; expression of adhesion molecules on the endothelial surface causes neutrophil adherence; production of factors that cause vascular dilatation Neutrophil activation and migration - expression of complementary adhesion molecules; increased motility - emigration from vessels into tissues; increased capacity for bacterial killing … |
|
|
|
Describe the development of acute inflammatory exudate
|
Stage 1: Early vascular changes –
1. dilation of vessels 2. adhesion of neutrophils to endothelium 3. fluid accumulation in interstitium Stage 2: Migration of neutrophils - neutrophils pass through vessel walls and migrate into perivascular CT. Stage 3: Early formation of exudate |
|
|
|
Describe (in general terms) the roles of immunoglobulins, complement cascade components and fibrinogen in the acute inflammatory process
|
Immunoglobulins: Opsonization, Complement activation
Complement: Opsonization, C3a, C5a, Membrane Attack Complex Fibrinogen: Fibrin framework for Bacterial immobilization, Neutrophil migration |
|
|
|
List the three components that form a typical acute inflammatory exudate
|
water and the dissolved solutes of blood including some or all plasma proteins, white blood cells, platelets and (in the case of local vascular damage) red blood cells.
|
|
|
|
Define lung consolidation
|
A region of lung tissue that, normally compressible, has filled with liquid. The fluid can be pulmonary edema, inflammatory exudate, pus, inhaled water, or blood.
|
|
|
|
List the three morphological types of acute inflammation
|
1. Suppurative or Purulent inflammation: Inflammation resulting in large amount of pus, which consists of neutrophils, dead cells, and fluid.
2. Fibrinous inflammation: Inflammation from a large amount of coagulated fibrolin. 3. Serous inflammation: effusion of non-viscous serous fluid, commonly produced by mesothelial cells of serous membranes, but may be derived from blood plasma. |
|
|
|
Describe suppurative inflammation and list at least 2 common conditions that demonstrate this type of inflammation
|
Suppurative or purulent (pus-containing) inflammation
Pus is a semi-liquid containing neutrophils, fluid and necrotic tissue An abscess is a circumscribed collection of pus |
|
|
|
Define pyogenic bacteria
|
Bacteria that promote purulent inflammation are called “pyogenic” bacteria
|
|
|
|
Describe fibrinous inflammation and list the typical locations for this type of inflammation
|
The exudate in fibrinous inflammation has a high plasma protein content
Fibrinogen, a plasma protein, is converted to fibrin and deposited in tissues. It is usually associated with serous membrane-lined cavities : pleural, pericardial and peritoneal Fibrin strands form a mat, often causing adhesion between adjacent surfaces |
|
|
|
Define and compare/contrast exudate versus transudate
|
Transudate
Extravascular fluid with low protein content and a low specific gravity. primary cell types are mononuclear cells: macrophages, lymphocytes and mesothelia cells. Exudate Extravascular fluid due to vessel alteration during inflammation (increased permeability, vascular constriction then dilation). High protein content, with cell debris present and high specific gravity. This is in contrast to transudate where the extracellular fluid is an ultrafiltrate of blood plasma and thus larger molecules such as proteins and cell debris are absent. |
|
|
|
Describe serous inflamation and name at least one condition that demonstrates this type of inflammatory response
|
Accumulation of fluid with a low plasma protein and cell content (transudate). Serous inflammation can be seen in the skin in response to a burn, and in serous membrane-lined cavities.
|
|
|
|
Describe the main factors that determine the outcome of acute inflammation
|
1) severity of tissue damage
2) capacity of stem cells within the tissue to replace the specialized cells required (regeneration) 3) the type of agent causing the damage |
|
|
|
Describe the process of healing by complete resolution following bacterial pneumonia or acute bronchitis
|
Can only occur when the connective tissue framework is intact and the tissue has the capacity to regenerate.
Examples: a. following pneumonia, regrowth of alveolar lining cells depends on resident stem cells to divide and differentiate. b. Recovery from sunburn (acute inflammatory response in the skin secondary to UV radiation) |
|
|
|
Compare and contrast acute abscess from chronic abscess
|
Abscess formation - acute inflammatory response fails to destroy/remove the cause of tissue damage; continues, usually with a component of chronic inflammation.
Acute inflammation progresses producing liquefaction of tissue to form pus An abscess cavity encapsulated by granulation and fibrous tissue is called a chronic abscess. |
|
|
|
List the main chemical mediators responsible for arteriolare dilation/vasodilation, increased vascular permeability and leukocyte activation and chemotaxis in the process of acute inflammation
|
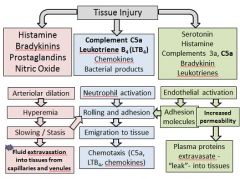
|
|
|
|
Describe the role of granulation tissue in wound healing and/or resolution of acute inflammation by fibrosis/scarring.
|
Healing by fibrosis (scar formation) - occurs with substantial damage to connective tissue framework and/or in tissues that lack ability to regenerate specialized cells
Necrotic debris and acute inflammatory exudate are first removed by macrophages The defect becomes filled by ingrowth of granulation tissue (called organization) Granulation tissue is gradually replaced by collagen to form a fibrous (collagenous) scar |
|
|
|
Describe the progression of changes in granulation tissue morphology leading to scar formation
|
Collagen deposition occurs over weeks. Collagen then remodels in response to tensile stresses. Fibroblasts regress and become relatively inconspicuous, as seen in this section (i.e. becoming fibrocytes).
Capillaries account for the red appearance of recent scars. Over months and years, the cellularity of the scar diminishes and there is progressive loss of capillaries - the scar contracts |
|
|
|
Compare and contrast wound healing by primary vs secondary intention
|
Primary intention involves epidermis and dermis without total penetration of dermis healing by process of epithelialization when wound edges are brought together so that they are adjacent to each other. Minimizes scarring
Secondary intention: The wound is allowed to granulate. Results in a broader scar and the healing process can be slow due to presence of drainage from infection |
|
|
|
List the hallmark co-existing morphological features of chronic inflammation
|
Co-existing tissue destruction, inflammation, and organization / repair are seen in chronic inflammation
|
|
|
|
Describe the three cell types which predominate in chronic inflammatory infiltrates
|
Lymphocytes: include both helper and cytotoxic T cells as well as B cells.
Macrophages: phagocytic and antigen-presenting. Plasma cells: differentiated B lymphocytes that produce antibodies. |
|
|
|
List the two major categories of chronic inflammation and describe their main causes
|
Non-specific - follows non-resolution of acute inflammation
Specific (primary) - response to certain specific types of injurious agents Non-immune mechanism – either granulomatous or non-granulomatous Immune mechanism – either granulomatous or non-granulomatous |
|
|
|
What are the common dyes used in staining?
|
Hematoxynin & Eiosin
Periodic Acid Schiff (PAS) for carbs. Toluidine Blue (Mast cells) Silver Stain - reticulin |
|
|
|
What type of Collagen is in Reticular fibers? In Hyaline Cartilage?
|
Hyaline - Type 2
Reticulin (a supporting mesh) - Type 3 |
|
|
|
What type of receptors do Osteoblasts and -clasts have?
|
Osteoblasts - PTH
Osteoclasts - Calcitonin |
|
|
|
Classify two types of bone
|
Primary - immature osteoids, abundant osteocytes, low mineral content, irregular collagen
Secondary: Mature osteoids, osteocytes in lacunae, High mineral content, parallel collagen, Haversian systems |
|
|
|
What are the two types of bone development?
|
Intramembranous - flat bones, developed in condensed mesenchyme
Endochondral - long bones. Cartilage replaced by bone. |
|
|
|
What are the ossification zones?
|
Epiphysis
Resting Zone Proliferative Zone Hypertrophic Zone Calcification Zone Ossifying Zone Diaphysis |
|
|
|
What types of helper cells are in the PNS
|
Satellite cells (cuboidal, surround the axon)
Schwann Cells |
|
|
|
What type of helper cells are in the CNS?
|
Ependymal cells (produce CSF)
Neuroglia: Astrocytes (nutrients) Oliodendrocytes (Myelin) Microglia (macrophages) |
|
|
|
How do you recognize the choroid plexus?
|
Pink debris
|
|
|
|
What causes Parkinson's Disease
|
Loss of Dopamine secreting cells in basal ganglia
|
|
|
|
What is Myasthemia Gravis?
|
autoimmune attack on acetylcholine receptors in neuro-muscular joints
|
|
|
|
What is Guillan-Barre?
|
Demyelinating disease in PNS affecting Schwann cells
|
|
|
|
What is multiple sclerosis?
|
Demyelinating disease in CNS affecting oligodendricytes
|
|
|
|
What is Wallerian Degeneration?
|
Degeneration of an axon distal to an injury
|
|
|
|
What part of the FAR covers proper source of supply?
|
Part 8
(FAR, Part 8) |
C
|
|
|
What are Nissle Bodies?
|
Rough ER and ribosomes
|
|
|
|
What are the layers in the cerebelum?
|
Pia Mater
1. molecular (neuroglial cells) 2. external granular (small pyramids) 3. exterenal pyramidal (medium pyramids) 4. internal granular 5, internal pyramidal (large pyramids) 6. multiform (axons) White matter |
|
|
|
What are the types of Lysosomes?
|
Primary - Unused
Secondary (phagosomes) - in use Residual Bodies - accumulated residues Lipofuscin - aging pigments |
|
|
|
Where are Lysosomal hyrdolases modified?
|
Covalently modified with M6P in the golgi. M6P receptor recognizes lysosomal proteins in the trans-golgi network for transport to lysosomes.
|
|
|
|
What do Peroxisomes contain?
|
Catalase which converts H2O2 to O2 and H2O. Very important in hepatocytes.
|
|
|
|
What phase of the cell cycle are stem cells in?
|
G0
|
|
|
|
What phase of the cell cycle are cardiac and nerve cells in?
|
Interphase
|
|
|
|
What two proteins regulate progress in the cell cycle?
|
Cyclins and CDKs. Cyclins bind CDKs which phosphorylate proteins pushing next stage
|
|
|
|
What are the different types of vesicular transport?
|
Exocytosis
Endocytosis - Receptor Mediated & Specific |
|
|
|
What is pinocytosis dependent on?
|
Actin
|
|
|
|
What do Parietal cells make?
|
HCl and IF
|
|
|
|
What do chief cells make?
|
Pepsinogen
|
|
|
|
What do Enteroendocrine cells make?
|
Gastrin, Secretin, CCK
|
|
|
|
Where are stem cells located in gastric ducts?
|
isthmus/neck
|
|
|
|
What do Mucous Neck cells make?
|
This, soluble secretion
|
|
|
|
What is the difference between serosa and adventitia?
|
Serosa - Peritoneal
Adventitia - Retroperitoneal |
|
|
|
What are the layers of the fundus?
|
Simple Columnar Epithelium
Lamina Propria Parietal cells chief cells muscularis mucosa |
|
|
|
What are the layers of the esophagus?
|
Stratified Squamous Epithelium
lamina Propria Muscularis Mucosae _____________ Tunica Mucosa Submucosa _____________ Tunica Submucosa Longitudnal muscle circular muscle ____________ Tunica Muscularis Externa Serosa ____________ Tunica Serosa |
|
|
|
What cell types do you find in each level in gastric pits?
|
Top - Serous Cells
Middle - Parietal Cells Neck - Serous Neck Cells Lower 1/3 - Enteroendocrine Cells Bottom - Chief cells |
|
|
|
What are the retroperitoneal organs?
|
S upra renal
A orta D uodenum P ancreas (not tail) U reters C olon (not trans or sigmoid) K idneys E sophagus R ectum |
|
|
|
What separates the three parts of the small intestine?
|
Duodenum - Plicae circulare, long vili, few goblet cells, brunner's glands
Jejunum - Plicae Circulare, long vili, more goblet cells, no glands Ilium - No plicae circulare, short vili, lots of goblet cells, peyer's patches ALL HAVE CRYPTS OF LIEBERKHUN |
|
|
|
What do Paneth cells make? What can you see in them?
|
Red granules = lysozyme (eiosinophilic)
|
|
|
|
What are the features of a villus?
|
Columnar epithelium with goblet cells, lamina propria inside with a lacteal.
|
|
|
|
What are M Cells?
|
Microfold - epithelial cells over peyer's patches that deliver antigens to lymphocytes
|
|
|
|
What is Pernicious Anemia?
|
Abscess or loss of parietal cells with low IF and B-12
|
|
|
|
What are the increasing levels of ducts?
|
Intercalated (squamous)
Striated (columnar) Intralobular (cuboidal) Interlobular Lobar (strat columnar) |
|
|
|
What are the three types of glands for the oral cavity?
|
parotid - branched acinar with serous units
sublingual - mucous acinar with serous demilunes Sumbandibular - both |
|
|
|
What are the two types of cells in the pancreas?
|
islet of langerhans in a CT capsule
Serous acinar glands with centro-acinar cells |
|
|
|
What are the two types of glands and what does each secrete?
|
Exocrine - compound tubular areolar w/serous secretion
Secretin CCK Endocrine - alpha (periphery) glucagon beta - center - insulin delta - dispersed - somatostatin |
|
|
|
What cues the contraction of the gallbladder and starts peristalsis?
|
CCK
|
|
|
|
What are the false lumens in the gallbladder called?
|
Rokitansky-Aschoff sinuses
|
|
|
|
What are the three "zones" of the liver lobules? What is carried in each?
|
Classic - portal vessels to central vein (blood)
Portal - central veins to portal vein (bile) Portal Acinus - Cells closest to portal vessels get nutrients first. Closest to central veins get them last |
|
|
|
What are kupfer cells?
Space of Disse? Ito Cells? |
Macrophages in the liver
Space where basal lamina should be Storage for Vit A |
|
|
|
What do hepatocytes lack that most cells have? What is special about the epithelium?
|
Basal lamina
It is fenestrated |
|
|
|
What are the three layers of blood vessels?
|
Tunica Intima (with internal elastic lamina)
Tunica Media (with external elastic lamina) Tunica Adventitia |
|
|
|
What are Weible-Palade bodies?
|
Bodies in blood vessels that contain Willebrand Factor and Factor VIII which release:
1) vasoactive sustances - Nitric Oxide for vasodilation Endothelin I for vasoconstriction Produce: Angeotensinase (ACE) Activated by cytokines to express cellular adhesion molecules They are NOT in capillaries. |
|
|
|
What is Von Willebrand's Disease?
|
Hereditary coagulation abnormality due to poor platelet cohesion. Not enough Weible-palade bodies.
|
|
|
|
What is P-Selectin?
|
a recruiting factor for WBC during injury
|
|
|
|
What distinguishes the different blood vessels?
|
Elastic Arteries - 40-70 layers of elastic lamelae
Muscular Arteries - easy to see IEL, 8-40 layers of lamelae, Vasa Vasorum Arterioles - 2-3 layers of lamelae. responsible for regulating blood pressure Capilaries - simple squamous epithelium |
|
|
|
What are the three types of capilaries?
|
Continuous, Fenestrated, Discontinuous/Sinusoidal
|
|
|
|
What is a post capillary venule?
|
High Endothelial Venules - vessels for lymphocytes to pass back and forth
|
|
|
|
How can you recognize veins? Bile ducts?
|
Veins - tunica adventitia is bigger than the tunica media. They are wider, more collapsible.
Bile ducts - very thick walls |
|
|
|
What is vasa vasorum?
|
small blood vessels serving large blood vessels
|
|
|
|
What are the different types of cell junctions?
|
Tight - zonula occludens - occludins
Intermediate - zonula adherins - vinculin Desmosomes - macula adherins - CAMs, desmoplakins Hemidesmosomes - integrin, laminin Gap - zonula communicans - connexon, made by 6 connexins |
|
|
|
Where do you find the different types of epithelium?
|
Simple Squamous - vessels, repiration, bowman's capsules, body cavities
Transitional - urinary tract Simple cuboidal - kidney tubules, thyroid, exocrine glands, germinal epithelium Stratified Squamous - epidermis, mouth, vagina Simple Columnar - GI tract, gall bladder Pseudostratified - Gi tract, gall bladder |
|
|
|
What is neoplasia in the epithelia? Glands? both?
|
Epithelia - Carcinoma
Glands - Adenoma Both - Adenocarcinoma |
|
|
|
What is Kartagener's Syndrome?
|
Immotile cilia without dynein arms which can't move mucous, leading to infection
|
|
|
|
What are Kinocilium?
|
9 + 0 microtubles used for sensory transduction
|
|
|
|
What makes up the basement membrane?
|
basal lamina + lamina reticularis
|
|

