![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back

Identify the Noble Gasses
|

|
|

Identify the Halogens
|

|
|

Identify the Alkaline Earth Metals
|

|
|

Identify the Alkali Metals
|

|
|

Identify the Transition Metals
|
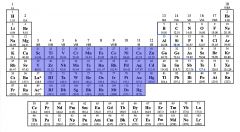
|
|

Identify the Inner Transition Metals
|
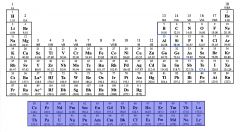
|
|

Identify the Lanthanoids
|
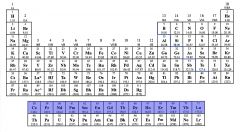
|
|

Identify the Actinoids
|
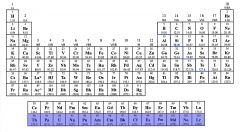
|
|

Identify the Main Group elements
|

|
|

Identify the Metalloids and Non-metals
|

|
|

What is the Halogen that will gain one electron to become isoelectronic with Neon?
|
Fluorine.
|
|
|
A positive ion is called a:
|
Cation
|
|
|
A negative ion is called a(n):
|
Anion.
|
|
|
If two atoms are isoelectronic, what does it mean?
|
They have the same number of electrons.
|
|
|
Cations tend to be formed by:
|
Metals
|
|
|
Anions tend to be formed by:
|
Non-metals
|
|

What ion is Calcium most likely to form? And what is it isoelectronic with?
|
2+ ion, isoelectronic with Argon.
|
|
|
What is special about the Noble Gasses and why does it occur?
|
The Noble Gasses are extremely stable and non-reactive due to their full outer orbit.
|
|
|
What is a period and what does it mean?
|
The periods are the rows (1-7) on the periodic table. The period number is equal to the number of occupied orbits.
|
|
|
What is a group and what does it mean?
|
A group is a column (1-18) on the periodic table. For main group elements, the group number represents the number of valence electrons. (Group 1; 1 valence electron. Group 17; 7 valence electrons)
|
|
|
How many electrons can occupy each orbit?
|
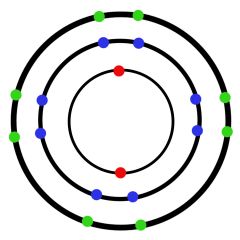
1st Orbit: 2
2nd Orbit: 8 3rd Orbit: 8 4th Orbit: 8 |
|
|
What is the mass of each; a proton, an electron and a neutron?
|
A proton and a neutron each have a mass of 1 u (atomic unit) and an electron has a mass of approximately 1/2000 u.
|
|
|
Can can an atom change from one element to another?
|
Yes, through decay.
|

