![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
34 Cards in this Set
- Front
- Back
|
A few review points of glycogen:
bond types What % of the glc are terminal ones? The ___ __ that can be added to has a protein cap called ____. |
α1,4 and at branches α1,6
8-10% Reducing end has glycogenin |
|
|
Why do polymerize glucose since it takes all this effort and enzymes instead of just shoving it in a storage container?
|
Glycogen is less osmotic to preserve water balance
|
|
|
About how much glycogen do we have at a time?
Where is it? What is the implication for glycogen storage diseases? |
1 lb-> still osmotic, so upper limit exists
In muscles and liver primarily, but it's in virtually every cell. hepatomegaly and cardiomegaly are common symptoms |
|
|
So where does excess glucose go if glycogen is capped?
|
GLUT2 transporter to liver, insulin tells liver to make triglycerides which are anhydrous, shipped out as LDL to adipose tissues for storage
|
|
|
Why do we branch glucose instead of keeping it linear?
|
More soluble in water
More free ends to depolymerize faster and get glc faster (In danger, need muscles -> need ATP -> need glc and need it fast -> branched you get a bunch of ends to take off. If it were linear, you'd only get one glc at a time) |
|
|
What is the glycogen in the liver used for?
What about in muscles? |
Liver uses stores to maintain blood [glc] especially between meals
Muscles, especially during exercise, use glycogen to act as readily available source for glycolysis within the muscle. |
|
|
How to make glycogen:
Prepping the glc pieces |
G6P <---phosphoglucomutase--->G1P (cytoplasm)
G1P + UTP <---UDP-Glc pyrophosphorylase-->UDP-Glc + PPi PPi --inorganic pyrophosphatase--> 2Pi |
|
|
Why is the PPi --> 2Pi step during UDP-Glc formation so important?
|
PPi is actually toxic
Makes reaction irreversible Le Chatlier principle says if product is removed, it drives the reaction forward |
|
|
How to make glycogen:
Adding glc to the glycogen |
UDP-Glc + C4 of glycogen ----glycogen synthase--> Glycogen + UDP
|
|
|
Rando Where all do you see pyrophosphate
|
DNA/RNA replication
protein synthesis with aminoacyl tRNA |
|
|
glycogen synthase
What all bonds does it catalyze? What size range of glycogen can GS work on? How tightly is this regulate? |
α1,4 only
all sorts of different sizes highly regulated |
|
|
How to make glycogen:
Making branches in your glycogen |
Branching enzyme takes 6-7 glc chain off a growing chain of 11-14 and makes an α1,6 linkage
|
|
|
What is the process of building glycogen called?
And the process of breaking it down? |
glycogenesis
glycogenolysis |
|
|
How do you start picking off glc from the ends of glycogen? What reaction is used?
|
use phosphorylase to cleave the α1,4 bonds until a chain of 4 glc remain, popping off G1P's (-phosphoglucomutase-> G6P-> glycolysis)
Cleave by phosphorolysis as opposed to hydrolysis since you use a Pi |
|
|
What are all the phosphorolysis reactions we've learned?
|
purine nucleotide phosphorylase PNP
phosphorylase for glycogenolysis |
|
|
After phosphorylase eats glycogen branches to 4, what happens?
|
Debranching enzyme takes all but one and moves it to a nearby terminal end. Then cleaves the α1,6 link between that last branch stub and the rest of the glycogen. This one is NOT phosphorylated.
|
|
|
What percent of glc released from glycogen is free glc instead of G1P?
|
8-10%
|
|
|
Where does glc activation happen?
Where does the actual glycogen building happen? Where does glycogen breakdown happen? Which specific membranes does glycogen go through? |
cytoplasm
cytoplasm cytoplasm None that we learned about. Too polar to traverse a lipid bilayer. |
|
|
Why is glycogenesis and glycogenolysis so important to regulate?
So where is it regulated? |
Otherwise the net reaction of pyrophosphorylase, inorg. pyrophosphatase, glycogen synthase, and phosphorylase would be UTP --> UDP + Pi = futile.
glycogen synthase and phosphorylase: the dominant enzyme of each pathway |
|
|
Describe the active and inactive forms of glycogen synthase.
|
Glycogen synthase I (active)
PKA for a specific Ser changing it to glycogen synthase D (inactive, PO4'd) phosphatase changes it back This is the opposite of phosphorylase. |
|
|
Describe the active and inactive forms of phosphorylase.
|
Phosphorylase b (inactive)
phosphorylase kinase acts on specific Ser changing it to Phosphorylase a (active, PO4'd) phosphatase changes it back This is the opposite of glycogen synthase. |
|
|
So net for glycogen synthase and phosphorylase: What does the presence/absence of PO4 mean for the breakdown/synthesis?
|
Protein phosphorylation: ups breakdown, downs synthesis.
Protein dephosphorylation: downs breakdown, ups synthesis |
|
|
What are target organs of epinephrine in terms of glycogen effects?
What receptor does epinephrine use? What does it do there? |
(results in phosphorylation)
Liver: ups glycogen breakdown for anaerobic muscular work Muscle: ups glycogen breakdown for glycolytic ATP formation These two are Cori Cycle. adrenergic receptor |
|
|
G-proteins are never ever ever ever a __
|
receptor. It can be coupled with a receptor. There can be G-protein coupled receptor.
|
|
|
What does glucagon do to regulate glycogen metabolism? Why is it doing this?
|
Liver: (results in phosphorylation) ups glycogen breakdown and gluconeogenesis (concurrently, not sequentially)
Glycogen will run out (12 hours at rest), so gluconeogenesis is important |
|
|
What does insulin do to regulate glycogen metabolism? Why is it doing this?
|
End result is phosphoprotein phosphatases which lower breakdown of glycogen and ups synthesis
|
|
|
Outline insulin pathway.
|
Tyr kinase class of receptor -> dimerizes -> autophosph on certain Tyr -> IRS docs -> other proteins like PI3K and RAS can dock and a phosphatase downstream turned on to change
|
|
|
Outline epinephrine/glucagon pathway to get to second messenger
|
Use adrenergic receptors- transmembrane, serpentine coupled to a G-:protein: Gs-GDP via α subunit
When hormone bound, GDP -swap-> GTP = Gs-GTP Gs αβγ-GTP drops βγ and Gs α-GTP goes to activate-> Adenylate cyclase. Membrane bound on internal side, this ATP -> cAMP + PPi (cAMP is the second messenger) Gs-GTP hydrolyzes itself after a certain time -> Gs-GDP hooks back up with βγ. |
|
|
What does cAMP do?
|
activates protein kinase A which acts on glycogen synthase I to change it to GS D
Phosphorylase kinase needs both [Ca2+ (SR) binds to calmodulin] (partially activates) and PKA to PO4 it (fully active) So "phosphorylated phosphorylase kinase is active and catalyzes phosphorylation of phosphorylase b to phosphorylase a (active form)" Easier to understand in a diagram than words. |
|
|
So, hormonal regulation of the enzymes ___ and ___ makes the hormones the ___ regulation of glycogen metabolism.
You can also ___ stimulate the one enzyme (_[name it]__) in ___ tissue only. |
glycogen synthase I/D and phosphorylase a/b
primary allosterically stimulate phosphorylase b in muscle tissue only |
|
|
Outline the allosteric regulation of phosphorylase.
Include location, the allosteric effector, and why it makes sense. |
In muscle only, high [AMP] makes higher phosphorylase b activity which makes sense because if [AMP] is high, you're obvi low on energy and need more glucose free-- works to activate PL b that was missed by phosphorylase kinase
Also-- [AMP] being high stimulates PFK1 to drive glycolysis. |
|
|
Shutting off glycogen breakdown:
What all do you need to modify? |
Hormones switch from epi, glucagon, cortisol to insulin
Gs-GTP -auto cleave-> Gs-GDP plus get beta gamma back. cAMP --phosphodiesterase-> AMP phosphoprotein phosphatase: phosphorylase kinase, phosphorylase a, glycogen synthase (activates) |
|
|
Describe the regulation of cAMP's phosphodiesterase.
|
It is not actually regulated but constitutively active. cAMP can work because it goes up in concentration enough to overcome it
|
|
|
Draw out the whole pathway that happens with glucagon/epinephrine signal.
Then modify after insulin signal received. |
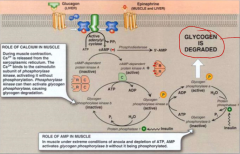
be sure to mark the locations where the hormones act.
Note: epinephrine's receptor is specifically deemed adrenergic (epi is catecholamine) Gs protein coupled receptor but receptors are the same. Hormone hitting receptor induces Gs-GDP -> Gs-GTP Add glycogen synthase being PO4'd to inactive by PKA (later with insulin phosphatase takes off) The glycogen that's released is G1P --phosphoglucomutase-> G6P -- LIVER ONLY G6phosphatase--> Glc (obviously muscle wouldn't want to do this) Add insulin's: Tyr kinase class of receptor -> dimerizes -> autophosph on certain Tyr residues-> IRS (insulin receptor substrate) docs -> other proteins like PI3K and RAS signaled to and phosphatase downstream turned on. cAMP cleaved by phophodiesterase (and it being inhibited by methyl xanthines: theophylline and caffeine) Mark where diseases interfere. Add actual glycogen building: G6P <---phosphoglucomutase--->G1P (cytoplasm) G1P + UTP <---UDP-Glc pyrophosphorylase-->UDP-Glc + PPi PPi --inorganic pyrophosphatase--> 2Pi branching enzyme. all of that. |

