![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
7 Cards in this Set
- Front
- Back
|
What is meant by rate of reaction? |
Measures how fast a reactant is being used up or how fast a product is being formed. Change inconcentration÷Time |
|
|
The rate of reaction is fastest at the start as each reactant is at its highest concentraion |
The rate of reaction slows down because reactant are being used up and their concentration decreases Rate of reaction is 0 once the reactants have been completely used up |
|
|
Name 4 things we can do to alter the rate of reaction. |
Concentration temperature use of catalyst Surface area of solid reactants |
|
|
What is the collision theory? |
Two reacting particles must collide for a reaction to occur. For successful reactions The particles collide with the correct orientation The particles have sufficient energy to overcome the activation energy barrier of the reaction |
|
|
How does pressure and concentration affect the rate of reaction? |
Increases the number of particles in the same volume the particles are closer together and collide more frequently therefore more effective collisions and increases rate of reaction. Pressure increase the number of particles in a given volume so same thing mentioned above. |
|
|
Methods for following the progress of a reaction. |
Monitoring the removal of the reactants. Monitoring the formation of a product. For example for gas Measure the gas produced in regular interval Or the loses of mass in reactants |
|
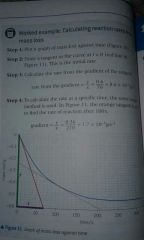
|
The Orange line is for any specific time. |

