![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
84 Cards in this Set
- Front
- Back
|
What are the 6 phases of B cell development and where do they occur?
|
1) generation of diversity in the bone marrow
2) elimination of self-reactive B lymphocytes 3) competition for secondary lymphoid tissue (going from marrow to secondary lymph tissue) 4) recirculation after maturation in secondary lymph tissue between lylmph, blood, and secondary lymph tissue 5) once Ag is found during circulation, activation and expansion 6) differentiation to Ab-secreting plasma cells and memory B cells that are left in secondary lymph tissue (pic) |
|
|
what are the stages of B cell development based on in general?
|
heavy and light chain position in process
|
|
|
what are the 7 stages of B cell development in the bone marrow?
|
1) stem cell
2) early pro-B heavy chain D-J rearrangement 3) late pro-B heavy chain V-DJ rearrangement 4) large pre-B test heavy chain with surrogate light chain. if acceptable (by external signals), then move on 5) small pre-B heavy chain proliferation and light chain VJ rearrangement 6) immature B heavy/light test with IgM expression first! 7) mature B is signified by IgD and IgM on the surface (pic) |
|
|
why are stem cells stimulated to become B cells?
|
stromal cells express VCAM molecules (cell adhesion molecules) and stromal cells release the cytokine IL-7
|
|
|
IL-7
|
stromal cell released cytokine which acts on B and T cells pushing them through development
|
|
|
what happens in B cell development if the heavy chain is not functional between the late pro-B and large pre-B steps?
|
1) the second allele will be queried to see if it will provide a suitable and functional H chain (2 tries)
2) if it will not, then apoptosis |
|
|
what happens in B cell development if the light chain is not functional between small pre-B and immature B cell steps?
|
1) there are two alleles for kappa gene and two for lambda gene that will be tested (4 tries)
2) if no functional light chain, then apoptosis |
|
|
what is the first and second checkpoints in B cell receptor development in the marrow?
|
1) heavy chain check point with surrogate light chain
2) heavy and light chain check |
|
|
what is the complete B cell receptor complex?
|
Ig and two invariant chains Ig-alpha and Ig-beta
|
|
|
what is a mature naive B cell?
|
mature B cell without antigen exposure
|
|
|
where do T cell precursors come from?
|
bone marrow -> blood -> thymus
|
|
|
how do you define primary lymph tissue?
|
where B and T cells mature (marrow and thymus)
|
|
|
where does the Ag presentation take place?
|
secondary lymph tissue
|
|
|
thymocytes
|
T cell residing within the thymus
|
|
|
how does location in the thymus correlate with T cell maturation?
|
progenitor T cells enter in the cortex and mature as they move toward the inner cortex and then to the medulla
|
|
|
what cytokine does the thymus secrete to encourage T cell maturation?
|
IL-7
|
|
|
what are the steps of T cell development in the thymus?
|
this process is really similar to B cell development
1) beta chain undergoes rearrangment 2) CD25 is released signifying TCR development 3) once proper rearrangment takes place (D-J then V-DJ), a test is done where beta chain is expressed with a surrogate alpha chain 4) this expression also brings upon a double positive state as well as the CD3 transduction protein 5) if the beta chain is good to go, then proliferation and alpha chain rearrangement begins 6) once alpha chain rearrangment is complete, a test is ran to see if it's good to go 7) if we like it, then we have a mature naive T cell that is double positive 8) TCR chooses sides (CD8 vs. CD4) based on first exposure to MHC I or MHC II |
|
|
what is double negative and double positive with respect to T cells?
|
double negative = neither CD4 or CD8 expressed
double positive = both CD4 and CD8 expressed |
|
|
what is the competition in T cell development?
|
alpha beta vs. gamma delta
|
|
|
what is "selection" of B and T lymphocytes?
|
getting rid of the self hostile B and T cells
|
|
|
self-reactive B lymphocytes
|
if a B lymphocyte reacts with bone marrow self-reactively, then the B cells won't cruise out of the marrow
|
|
|
what types of self-Ag will B cells interact with to test self-reaction?
|
1) multivalent self-antigen that will lead to apoptosis
2) soluble self-antigen that leads to peripheral migration and inactivation (anergic B cells) |
|
|
tolerance with respect to B and T selection
|
when immature B and T cells stop reacting to self antigen
|
|
|
what is a way to get rid of self-reactive B cells without apoptosis?
|
receptor editing. RAG activity can happen late in the game to rearrange to make something that is not self-reactive. this only happens on the light chain and with B cells that interact with the multivalent self-Ag.
|
|
|
what are significant signals that you have a mature, naive B cell?
|
1) no more RAG-1, RAG-2 expression
2) IgM and IgD expression 3) migration to secondary lymph tissue 4) competition to enter secondary lymph tissue 5) after entering, the B cell will exit and circulate to find Ag |
|
|
is interaction with an Ag enough to cause an immune response with a B cell?
|
no. a T cell must be there to activate a B cell. if a T cell does not activate, then the B cell will die or be anergic. this is another way to avoid self-reaction
|
|
|
what is the difference between positive and negative selection?
|
(+) - self restriction where Thymocytes must recognize self MHC presented by cortical epithelial cells. if they cannot, then they will undergo apoptosis
(-) - must be self tolerant. they cannot be activated by self antigen presented by dendritic cells |
|
|
how is self tolerance established in periphery with T cells?
|
there must be a co-stimulatory agent in the periphery and if it doesn't exist, then it undergoes apoptosis or becomes anergic.
|
|
|
what does CD8+ T cell become when properly stimulated?
|
cytotoxic T cell
|
|
|
what are CD4 Th1 cells activated by?
|
macrophage which will release cytokines
|
|
|
what are CD4 Th2 cells activated by?
|
B cells which will release antibodies
|
|
|
what are the three effector T cells?
|
CD8, CD4 Th1, CD4 Th2
|
|
|
why do T cells stop and initially connect to an APC in secondary lymph?
|
LFA-1 on the T cell attaches to the ICAM-1 on the APC to stop long enough to test the TCR-MHC complex to see if the correct Ag is being presented. if it is, then a conformational changed occurs in the LFA-1 that clamps down to hold onto this "docking"
|
|
|
what happens when a T cell encounters a matching antigen in the secondary lymph?
|
1) activation ->trapped
2) proliferation 3) differentiation 4) 5 days later, they can leave the secondary lymph and circulate to find more Ag |
|
|
what is the signal that promotes proliferation and differentiation?
|
CD28 on the T cell
B7 on the APC this secondary signal activates t cell! very important! |
|
|
what is the series of intracellular signaling that happens when a TCR and MHC bind with a matching Ag?
|
1) Ag binds
2) phosphorylation of ITAMs which are on the cellular side of the CD3 transmembrane supportive molecule 3) now ITAM tails can be recognized by the sarchomology domain |
|
|
T cell proliferation
|
IL-2 is produced by the T cell and provides self-stimulated proliferation
fortunately, whenever a T cell is activated, IL-2R (receptor) is made, too. IL-2 is a self-stimulated autocrine proliferation stimulating complex |
|
|
T cell differentiation
|
differentiated CD4 T cells via certain cytokines go to:
1) TFG-beta -> Tregs 2) IL-12, IFN-gamma -> Th1 3) IL-4 -> Th2 |
|
|
what cytokines do Th2 cells produce?
|
IL-4, IL-5
|
|
|
what cytokines do Th1 cells produce?
|
IL-2, IFN-gamma
|
|
|
activating CD8 T cells without dendritic cells
|
1) CD4 T cell activates macrophage -> upregulates B7 -> CD8 activation
2) CD4 T cell activated by macrophage -> IL-2 produced by CD4 activates CD8 |
|
|
what's the difference between the effector T cell and the naive T cell?
|
effector does not need co-stimulation via B7:CD28 for it to be activated
|
|
|
what 2 things does IFN-gamma do?
|
1) inhibits replication of viruses
2) increases expression of MHC I on other infected cells |
|
|
how are CD8 cytotoxic T cells activated (from undifferentiated T cells)?
|
dendritic cells are ideal because of high MHC levels and B7
|
|
|
how does cytotoxic T cell identify antigen presenting cell that is not a professional APC?
|
CTL (cytotoxic T cell) releases lytic granules containing cytoxoins, perforins, granzymes
|
|
|
how do CTLs specify where the cytotoxins will be effective? why don't they kill surrounding cells?
|
immunesynapse - tight junction between CTL and antigen presenter by connection of LFA (on CTL) to I-CAM (on APC)
|
|
|
how can a CTL kill many cells at a time?
|
it can't! it kills one at a time with a specific immunesynapse for each kill. bam!
|
|
|
what cytokine is produced that stops virus replication and gives neighboring cells the heads up to express MHC-I if they are infected?
|
IFN-gamma
|
|
|
how do Th1 cells activate macrophage?
|
1) identify macrophage expressing MHC-II (primary signal)
2) Th1 express CD40 ligand that binds to CD40 on macrophage (this is a necessary secondary signal) to activate the macrophage 3) CD40:CD40 ligand causes IFN-gamma to be expressed by macrophage to complete activation 4) effector cytokines are further released: IFN-gamma, CD40 ligand, IL-2 |
|
|
how do Th2 cells activate B cells?
|
1) identify B cell expressing MHC-II (primary signal)
2) Th2 express CD40 ligand that binds to CD40 on macrophage (this is a necessary secondary signal) to activate the B cell 3) effector cytokines are further released: IL-4, IL-5, CD40 ligand 4) B cell differentiates into final effector cell: Plasma cell |
|
|
Memory T cells
|
a small group of CD4 and CD8 T cells that are exposed to IL-7 and IL-15 that are reactive to a specific antigen and are stored for later use. they remain inactive until the specific Ag shows its face again.
|
|
|
what are the two main signals that allow naive B cells to be activated in a T-dependent activation?
|
2 signals:
1) BCR interacts with epitope and presents to CD4+ TCR on the Th2 2) CD40:CD40L co-stimulatory |
|
|
TD antigens
|
thymus dependent antigens - antigens that requires T cell involvement (Th2) for the B cell to be activated to handle such an Ag
|
|
|
TI antigens
|
thymus independent antigens - antigens that do not require T cells for B cell activation. 2 catagories
1) TI-1 2) TI-2 |
|
|
what are the two roles of the BCR in T-dependent B-cell activation?
|
1) binding antigen
2) internalize antigen by endocytosis and process antigen to present to Th2 |
|
|
even if the B cell processes and presents and antigen, what is required in order for it to undergo proliferation and differentiation to become antigen secreting plasma cells?
|
T cell activation by the CD40:CD40L interaction
|
|
|
in B cell activation, what is the equivalent to the CD3 phosphorylation and cascade in T cell activation?
|
transmembrane ITAM phosphorylation in the B cell
|
|
|
what composes the B-cell co-receptor complex?
|
CD19, CD21 (CR2), CD81
|
|
|
what is the B-cell co-receptor complex used for?
|
TI antigen activation when C3d complement protein (which at this point has opsonized and is bound to the pathogen) binds to the B-cell co-receptor (CR2 specifically) providing the necessary secondary signal for activation
|
|
|
TI-1 antigens
|
aka mitogens like LPS in cell walls which will activate the B-cell. this provides the primary signal for TI B-cell activation
|
|
|
TI-2 antigens
|
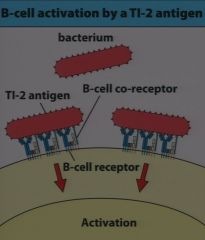
large repeating polysaccharide molecules on the outside of a pathogen that activate several Igs on the surface of the B cell. this provides the primary signal for TI B-cell activation
(pic) |
|
|
what happens in the follicle (B-cell zones) of the lymph node?
|
1) B cells differentiate into memory cells and plasma cells
2) B cells undergo somatic hypermutation (increase diversity to increase affinity for Ag) 3) isotype switching occurs |
|
|
what are centroblasts?
|
proliferating B cells in the lymph node follicle
|
|
|
what happens if in the follicle hypermutation produces an Ig with lower affinity to the antigen?
|
the cell is killed off.
|
|
|
what are centrocytes?
|
B cells in the lymph node follicle that are attempting to increase affinity by hypermutation, but have not yet been affirmed to have better affinity.
|
|
|
how is the affinity of a centrocyte tested?
|
follicular dendritic cells express complement receptors that bind the Ag of interest and the centrocytes tries to bind the Ag. only the centrocytes with higher affinity become plasma cells.
|
|
|
what cytokines promote IgG isotype switching?
|
IL-4, IFN-gamma, TGF-beta
|
|
|
what cytokines promote IgE isotype switching?
|
IL-4
|
|
|
what cytokines promote IgA isotype switching?
|
TGF-beta, IL-5
|
|
|
what is the plasma cell?
|
effector B cell that secretes antibodies
|
|
|
what is the first wave and second wave of the primary response?
|
first wave: Th2 cells activate B cells, B cells become plasma cells
second wave: B cells hypermutate to increase affinity in follicles, B cells with highest affinity become plasma cells within the bone marrow for safe keeping |
|
|
where do memory B cells come from?
|
another destination of centrocyte differentiation for subsequent infections
|
|
|
what are the major defenses against intracellular and extracellular pathogens, respectively?
|
intra - CTLs
extra - Abs |
|
|
what two examples of pathogenic toxins cause disease and are fought with secreted Abs?
|
tetanus and diphtheria
|
|
|
where are FcR (Fc receptors) in the immune response and what is their purpose?
|
FcR bind Fc regions of secreted Abs once they have opsonized or bound an Ag. FcRs are located on phagocytizing cells. FcR:Fc binding enhances phagocytosis.
|
|
|
What is the primary purpose of IgG?
|
the dominant blood-borne antibody that is secreted and moves as a free Ab.
|
|
|
How are IgGs extravasated?
|
FcR for IgG is the Brambell receptor (FcRB)
1) IgG Fc binds to FcRB on blood vessel lumen side of epithelial cell 2) transcytosis process moves IgG through cell to tissue side of cell 3) IgG is released into extracellular space |
|
|
How are IgM limited?
|
they cannot undergo extravasation and move from blood to tissue?
|
|
|
what form is IgA always found in?
|
dimeric IgA
|
|
|
How does IgA get into the mucus in the lumen of the GI (or any surface with a mucosal layer)?
|
1) dimeric IgA binds poly-Ig receptor on basolateral side of epithelial cells
2) transcytosis moves IgA dimer to apical surface (lumenal surface) 3) "secretory piece" molecule anchors IgA dimer to apical surface of epithelial cell so it won't wash away |
|
|
What Ig does fetus receive from mother in utero?
|
IgG
|
|
|
What Ig does neonate receive from mother's milk?
|
dimeric IgA
|
|
|
What's the deal with IgG and neonates?
|
IgG is given to the fetus pre-birth. After birth, the neonate does not produce it's own IgG immediately, so there is a one year lag before IgG can be produced at significant levels. This is why dimeric IgA is very important to get from mom!
|
|
|
What is the significance of IgM in neonates?
|
It is produced internally as a fetus and then even more so at birth and fills some of the gap left by little IgG and little IgA at birth.
|

