![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
44 Cards in this Set
- Front
- Back
|
Therapeutic Index
|
ratio of the dose that produces toxicity to the dose that produces a clinically desired or effective response in an experimental population.
Therapeutic Index = TD50/ED50 or LD50/ED50 |
|
|
What must the Therapeutic Index (safety margin) be in order for a drug to be usable?
|
must be at least 1, but ideally will be much greater than 1.
ex. Digoxin has a TI of 2 Penicillin has a TI of > 100 |
|
|
Therapeutic range (probability based)
|
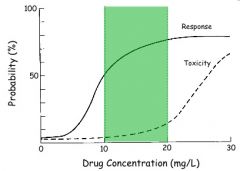
ideally large range of drug concentration where efficacy exists (starts at EC50), but toxicity does not (ends where toxicity effects begin to increase).
note that since Emax, EC50, and n are different for all individuals, the therapeutic range is good for MOST patients |
|
|
What is the significance of the efficacy of Theophylline?
|
> 5 ug/ml = bronchodilatory effect
10-20 ug/ml = TR for anti-asthmatic (target 10-15) 5-10 ug/ml = TR for apnea therapy in children (target 7.5) different therapeutic ranges for different indications |
|
|
What is the significance of the toxicity of Theophylline?
|
5-20 ug/ml - headache, sausea, tremor, sleeplessness
15-35 ug/ml - vomiting, diarrhea, stomach pain, nervousness, tachychardia > 35 ug/ml - convulsions, cerebral hypoxia, arrhythmia, cardiac arrest, death |
|
|
What is the defined therapeutic range of Theophylline?
|
10-20 ug/ml
|
|
|
Type A adverse drug reactions (ADR)
|
"side effects"
dose-related, predictable, expected, accepted to occur |
|
|
Type B adverse drug reactions (ADR)
|
non-dose related, unespected, allergic reaction, carcinogenic, teteratogenic, idiosyncratic
|
|
|
What are all the types of ADR (adverse drug reactions?
|
Type A - side effects
Type B - bizarre effects Type C - chronic effects Type D - delayed effects Type E - end-of-treatment effects A and B are the most important and will be discussed in detail |
|
|
What is idiosyncratic?
|
individually peculiar with respect to known character
|
|
|
What affects the frequency and severity of Type A ADRs?
|
therapeutic range and selectivity
|
|
|
What is the difference between silenafil, vardenafil, and tadalafil?
|
All are phosphodiesterase inhibitors for the treatment of erectile dysfunction.
silenafil - viagra vardenafil - levitra tadalafil - Cialis The difference is that there are PDE1 - PDE11 throughout the body and they all target PDE5 with the same potency, but have different potency for other PDEs, so they offer different side effects because of the altered potency. |
|
|
What affects the frequency and severity of Type B ADRs?
|
genetics of an individual
|
|
|
Common examples of Type B ADRs
|
1) Hepatotoxicity because of toxic intermediates that some individuals are sensitive to
2) Nephrotoxicity 3) Pancreatitis 4) Hypersensitivity I, II, III, and IV (allergic) reactions - immunologically mediated manifesting as urtikaria, rhinitis, bronchial asthma or angioedema |
|
|
What is the mechanism of a Type I hypersensitivity reaction and what Ab mediates this reaction?
|
IgE mediated, anaphylactic reaction and Ag-Ab reaction causing systemic anaphylaxis, caused by penicillin and cephalosporins
|
|
|
What is the mechanism of a Type II hypersensitivity reaction and what Ab mediates this reaction?
|
IgG, IgM mediated cytotoxic, Ag-Ab reaction producing hemolytic anemia caused by penicillin and quinine
|
|
|
What is the mechanism of a Type III hypersensitivity reaction and what Ab mediates this reaction?
|
IgG, IgM mediated immune complex or immune complex-Ab reactions producing serum sickness caused by penicillin, cephalosporins, isoniazid, pheytoin
|
|
|
What is the mechanism of a Type IV hypersensitivity reaction and what Ab mediates this reaction?
|
T-cell mediated delayed hypersensitivity which takes > 12 hrs to respond with Ag-T-cell interactions producing topical (not systemic) problems. usually rashes
|
|
|
tolerance
|
diminishing effect to a repeated stimulus (drug in dose). can be metabolic or functional.
|
|
|
metabolic tolerance
|
time-dependent change in PK parameters (clearance)
|
|
|
functional tolerance
|
time-dependent change in pharmacodynamic parameters such as
1) decrease in receptor number or 2) receptor desensitization |
|
|
graphically, how does functional tolerance present?
|
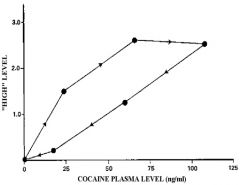
as a clockwise hysteresis loop
|
|
|
What phenomenon does ranitidine provide in pt populations?
|
functional tolerance with constant rate infusion not being able to provide constant correlated 1:1 control over gastric pH
|
|
|
receptor desensitization
|
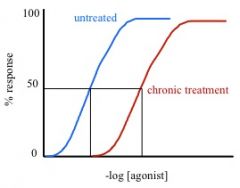
a cause of functional tolerance where receptor number does not change, but affinity for agonist is reduced, resulting in a rightward shift of the binding curve. transduction is reduced and effect changes.
|
|
|
does Emax change in receptor desensitization?
|
nope, the curve is just shifted to the right because more [agonist] is needed.
|
|
|
receptor down-regulation
|
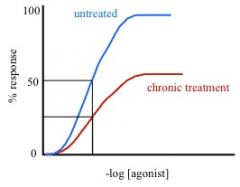
a cause of functional tolerance where proteolytic degredation of receptor occurs. receptors are interalized by use of endosomes and lysosomes -> net loss in total receptors -> Emax decreases, but Kd remains the same. makes sense.
|
|
|
tachyphylaxis
|
special case of functional tolerance. happens rapidly (minutes to hours) and is rapidly reversible.
|
|
|
What are two examples of tachyphylaxis?
|
1) indirect sympathomimetics (Amphetamines, Ephedrin) because the repeated exposure depletes neurotransmitter storage. these drugs simple cause release of these NTs. when they run out. they run out. give it some time, and they will come back.
2) Nitrates (Nitroclycerin) - converting nitrates to NO (the whole point of nitrates) uses SH-groups that are of limited supply. once the SH pool is replenished, the nitrates will regain their potency |
|
|
dependence
|
1) rewardive psychological dependence - if it feels good, do it
2) withdrawal physiological dependence - it feels bad if you don't do it |
|
|
neuroadaptation related to dependence
|
adaptive mechanisms initiated by the brain to restore physiological homeostasis when a drug is introduced that can lead to physiological dependence
|
|
|
receptor down regulation related to dependence
|

happens with an agonist drug
1) receptor down regulation with repeated exposure 2) recovery after removal of drug where receptors must slowly up-regulate (withdrawals) |
|
|
Three ways that receptors respond that effects dependence.
|
1) downregulation
2) affinity 3) upregulation |
|
|
receptor up regulation related to dependence
|
happens with an antagonist drug
1) antagonist blocks normal action 2) cell up regulates to provide receptors for normal ligand interaction 3) when drug is removed cell must slowly down regulate to return to homeostasis |
|
|
affinity related to dependence
|
affinity changes with use of drug
1) receptor changes affinity in order to interact with drug, but changes so that is not interactive with normal ligand 2) drug is removed and receptor can no longer interact with normal ligand 3) affinity must change to be viable to use with normal ligand when drug is gone for a length of time |
|
|
what happens with cyclosporin in pediatric pharmacodynamics?
|
as age increases, the EC50 increases which reflects a decreased efficacy relative to dose as age increases
|
|
|
what happens with warfarin in pediatric pharmacodynamics?
|
the effect is 2x in pediatrics which corresponds to potency and sensitivity is 2x in pediatrics compared to adults
|
|
|
what happens with Sotalol in pediatric pharmacodynamics?
|
effects of the drug are neonates > infants > adolescents
|
|
|
elderly
|
elderly are more sensitive to drugs, rather because of receptor differences, their sensitivity is different. the examples that he gave showed a higher sensitivity
|
|
|
FDA food and drug administration
|
established in 1938 by Federal Food Drug and Cosmetic Act
|
|
|
3 roles of the FDA
|
1) New drug application (NDA)
2) Abbreviated new drug application (ANDA) - for generic or bio-equivalence of preexisting drugs 3) Investigational New drug application (IND) - first time use in humans |
|
|
of every 10,000 compounds submitted to the FDA how long does it take and how many are accepted?
|
~15 years for 1 drug
|
|
|
What are the preclinical stages of producing a drug through the FDA?
|
1) discovery
2) preclinical dev I - animal in vivo and in vitro trials, manufacturing procedure established 3) preclinical dev II - establish PK and PD relationships and animal toxicology trials in rodent and nonrodent categories (mouse and monkey) at least 2 species, risk assessment, plan for human trial 4) Preclinical to clinical transition - IND application 5) Clinical Phase 1 - <100 subjects, efficacy is not an objective, just introducing it into human targets 6) Clinical Phase 2 - perform in pts with illness, prove therapeutic value, identify best demographic and dosing regimens 7) Clinical Phase 3 - |
|
|
what is the transition between preclinical and clinical phases of drug development?
|
4) Preclinical to clinical transition - IND application
|
|
|
What are the clinical stages of producing a drug through the FDA?
|
5) Clinical Phase 1 - <100 subjects, efficacy is not an objective, just introducing it into human targets
6) Clinical Phase 2 - perform in pts with illness, prove therapeutic value, identify best demographic and dosing regimens 7) Clinical Phase 3 - confirm efficacy and safety >1000 pts, detect adverse reactions with two independent double blind randomized placebo controlled trials, fill out NDA 8) Clinical Phase 4 - post approval, marketing, refine efficacy in different populations, further adverse reaction study, |

