![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
|
What are the three phases that a cell goes through relative to stress?
|
1) adaptation
2) injury (reversible or irreversible) 3) death through necrosis or apoptosis |
|
|
Atrophy
|
an adaptation response to a cell where the size of the cell is decreased. causes: decreased workload, ischemia, lack of hormonal or neural stimulation, malnutrition
|
|
|
Hypertrophy
|
an adaptation response to a cell where the size of the cell is increased. causes: increasd workload, increased hormonal stimulation
|
|
|
Hyperplasia
|
an adaptation response to a cell where the number of cells increases.
|
|
|
hormonal hyperplasia
|
increases functional capacity of tissue like breast hyperplasia during puberty
|
|
|
compensatory hyperplasia
|
increases tissue mass after damage or resection, like regeneration of liver or kidney after partial removal
|
|
|
pathologic hyperplasia
|
usually caused by excessive hormonal stimulation and will regress when hormonal stimulation is stopped.
|
|
|
example of pathologic hyperplasia
|
thyroid hyperplasia in Graves Disease
|
|
|
Hypoplasia
|
underdeveloped organ because of deficient number of cells, commonly caused by congenital condition
|
|
|
Aplasia
|
complete lack of development of organ
|
|
|
Examples of hypoplasia
|
testes in Klinefelter's, pulmonary hypoplasia in newborns with oligohydramnios
|
|
|
Examples of aplasia
|
thymus in DiGeorge, cutis aplasia, anemia
|
|
|
metaplasia
|
reversible change in which one type of cell is replaced by another. usually caused by stress that causes a change and can be problematic when stressor does not desist. eg epithelial metaplasia in smokers
|
|
|
dysplasia
|
"disordered growth"
|
|
|
hypoxia
|
cell injury caused by decreased oxygen to tissue usually because of decreased blood flow
|
|
|
physical agents that can cause cell injury
|
trauma, temperature extremes, radiation, electrical shock
|
|
|
chemical agents that can cause cell injury
|
hypertonic glucose, salt, poison, pesticides, alcohol, drugs, oxygen in high concentrations
|
|
|
infectious agents that can cause cell injury
|
viruses, bacteria, fungi, parasites
|
|
|
immunologic reactions that can cause cell injury
|
anaphylaxis, autoimmune disease
|
|
|
genetic derangements that can cause cell injury
|
enzyme defects, chromosomal abnormalities
|
|
|
four sites of cell injury
|
1) \/ ATP
2) Membrane 3) /\ intracellular Ca 4) ROS |
|
|
what are the effects of \/ ATP?
|
1) ER swelling, cellular swelling because of Na/K pump cannot be regulated;
2) \/ glycogen, /\ lactic acid because now using glycolysis; 3) lipid deposition |
|
|
what are the effects of /\ Ca+
|
ATPase activity increases
phospholipases break down membranes proteases break down proteins endoluceases make acid increases mitochondrial permeability -> apoptosis |
|
|
mitochrondrial damage -> ?
|
\/ ATP, /\ Ca+
|
|
|
list ROS that could be problematic for cell damage?
|
O2-, H2O2, OH-
|
|
|
why are ROS problematic?
|
disrupt lipid membranes, oxidative modification of proteins, DNA damage
|
|
|
how can one defend against ROS damage?
|
1) antioxidants (vit A, C, E, glutathione)
2) storage and transport proteins (cerulupolasmin and transferrin) bind Fe and Cu to stop formation of ROS 3) intracellular enzymes (superoxide dismutase, catalase, glutathione peroxidase) convert ROS to O2 and H2O |
|
|
What are the 2 types of cell death?
|
necrosis and apoptosis
|
|
|
What is significant about necrosis?
|
always pathologic and does not just refer to cell death, but also the spectrum of morphological changes that follow cell death in living tissue
|
|
|
what cytoplasmic changes occur with necrosis?
|
1) eosinophilia - eosin binds to cytoplasmic proteins and causes brilliant deep pink staining
2) hyalinization - glycogen loss causes a homogenous cytoplasm instead of granulated 3) vacuolization - enzymatic digestion of organelles leaves vacuoles 4) calcification |
|
|
what nuclear changes occur with necrosis?
|
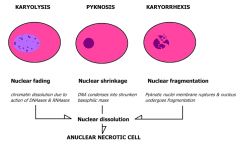
1) karyolysis - nucleus is very pale blue
2) pyknosis - nucleus shrinks and becomes darker 3) karyorrhexis - nucleus is fragmented |
|
|
what are the three microscopic and gross patterns of necrotic tissue?
|
1) coagulative
2) liquefactive 3) caseous |
|
|
coagulative necrosis
|
tissue pattern of necrosis that is most common. nuclei absent, but cell outlines still exist. common in ischemic death of heart, liver, and kidney tissue.
|
|
|
liquefactive necrosis
|
tissue pattern of necrosis where cells liquefy and dissapear microscropically and grossly (gross holes in tissue), common with bacterial and fungal infections because of hypoxic death in CNS in brain infarcts, pancreas, abscesses
|
|
|
caseous necrosis
|
combination of liquefactive and coagulative, presents grossly as a cheesy appearance, microscopically as necrotic focus surrounded by granulomatous inflammation, common in TB
|
|
|
what are the other lesser discussed catagories of necrosis
|
fat necrosis, fibrinoid necrosis, wet or dry gangrenous necrosis
|
|
|
apoptosis
|
programmed cell death can be physiologic or pathologic, caused by fragmentation of INDIVIDUAL cell nuclei and cytoplasm; never elicits inflammation due to rapid phagocytosis
|
|
|
mophological quality of apoptosis
|
1) involves single cells
2) nuclear chromatin condenses against nuclear membrane 3) blebbing (pooching of cell membrane) into extravessicles to be phagocytized 4) does not elicit inflammation |
|
|
normal lysosomal catabolism
|
molecule is endocytized by cell, lysosome internally endocytizes the foreign body, catbolises the foreign body, binds and excises the bits of the foreign body outside the cell and then lysosome restores itself to take care of another foreign body
|
|
|
abnormal lysosomal cataolism
|
for whatever reason, lysosomes fail and there is intracellular accumulation that leads to apoptosis
|
|
|
lipid accumulations
|
intracellular accumulations of lipids that can be pathologic. fatty liver can develop and can be visualized by Oil Red O Staining
|
|
|
other intracellular lipid accumulations
|
atherosclerosis, xanthomas, inflammatory foci, cholesterolosis, Neimann-Pick disease
|
|
|
intracellular protein accumulation
|
presents as rounded eosinophilic droplets or aggregates in cytoplasm,
|
|
|
exogenous pigments
|
common with carbon or coal dust, tattoos
|
|
|
endogenous pigments
|
melanin, lipofuchsin (yellow or brown "aging pigment" or bruising pigment, hemosiderin (derived from hemoglobin, yellow brown bruising pigment), bilirubin (from bile, leads to jaundic)
|
|
|
what are the two types of calcification?
|
1) dystrophic - very comon, Ca deposits in damaged tissue (heart valves in elderly)
2) metastatic - uncommon calcium deposits in normal tissues |

