![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
|
Organic Chemistry:
|
The chemistry of the compounds of carbon
|
|
|
Carbon Compounds include:
|
- DNA (contains our genetic info)
- proteins that catalyze all the reactions in our body and that constitute the essential compounds of our blood, muscles, and skin - combine with oxygen, carbon compounds furnish the energy that sustains life. |
|
|
What is "vitalism" belief and is it still valid?
|
- The intervention of a "vital force" was necessary for the synthesis of an organic compound.
- such synthesis could only take place in living organisms - theory ended up being false.... 1828-1850 # of compounds that were clearly organic were synthesized from sources that were "inorganic") |
|
|
Structural Theory:
|
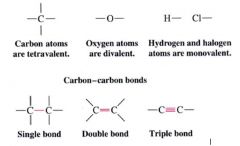
1. atoms in organic compounds can form a fixed # of bonds using their outermost shell (valence) electrons
2. carbon atom can use one/more of its valence electrons to form bonds to other carbon atoms |
|
|
Kekule's definition of organic chemistry:
|
a study of the compounds of carbon
|
|
|
Isomers:
|
different compound that have the same molecular formula
|
|
|
constitutional isomers;
|
different compounds that have the same molecualr formula but differ in their connectivity, that is, in the sequence in which their atoms are bonded together.
- usually have different physical properties - different chemical properties |
|
|
Ionic Bonds:
(electrovalent) bond |
formed by the transfer of one or more electrons from one atom to another to create ions.
|
|
|
covalent bonds:
|
bond that results when atoms share electrons
|
|
|
Octet rule:
|
tendency for an atom to achieve a configuration where its valence shell contains 8 electrons
|
|
|
Ions:
|
atoms that gain / lose electrons and from charged particles
|
|
|
ionic bond:
|
an attractive force between oppositely charged ions
|
|
|
Electronegativity:
|
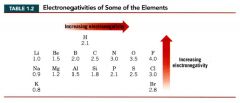
a measure of the ability of an atom to attract electrons.
- increases as we go across a horizontal row of the periodic table from left to right - increases as we go up a vertical column. |
|
|
cation:
|
+
|
|
|
anion:
|
-
|
|
|
why do the ions form?
|
based on the lewis-kossel theory:
both atoms achieve the electronic configuration of a noble gas by becoming ions. |
|
|
Salts:
|
ionic compounds form only when atoms of very different electronegativities transfer electrons to become ions.
|
|
|
Covalent bonds:
|
- when two/more atoms of the same/similar electronegativities react
- complete transfer of electrons doesn't occur - products of covalent bonds are called molecules. |
|
|
valence electrons:
|
electrons of an atom's outermost shell
|
|
|
simple rules that allow us to draw proper lewis structures:
|
1. show the connections between atoms in a molecule or ion using only the valence electrons of the atoms involved.
2. For main group elements, the number of valence electrons a neutral atom brings to a Lewis structure is the same as its group number in the periodic table. 3. If the structure we are drawing is a negative ion (an anion), we add one electron for each negative charge to the original count of valence electrons. If the structure is a positive ion (a cation), we subtract one electron for each positive charge. 4. try to give each atom the electron configuration of a noble gas. Draw structures where atoms share electrons to form covalent bonds or transfer electrons to form ions. - to achieve an octet of valence electrons, elements such as nitrogen, oxygen, and the halogens typically share only some of their valence electrons through covalent bonding. |
|
|
why do atoms share electrons?
|
- obtain the configuration of an inter gas
- sharing electrons produces increased electron density between to positive nuclei. - resulting attractive forces of nuclei for electrons is the "glue" that holds the atoms together. |
|
|
how to calculate the formal charge on a particular atom?
|
1. we assign the number of valence electrons associated with the relevant atom
a. half of all the electrons that are in covalent bonds to it b. all of its unshared electron pairs 2. use the periodic table to determine the the number of valence electrons that the atom would have in its neutral unbounded state. 3. compare the number of valence electrons assigned to the atom in its bonded state with the number of valence electrons in its neutral unbounded state a. if the atom in the lewis structure is assigned fewer electrons than it would have as a neutral unbonded atom, atom has a positive formal charge with magnitude corresponding to the number of electrons in the difference. b. if the atom in the Lewis structure is assigned more electrons than it would have as a neutral unbounded atom, the atom has a negative formal charge with magnitude corresponding to the number of electrons in the difference. 4. the arithmetic sum of all the formal charges in a molecule or ion will equal the overall charge on the species. |
|
|
Methods of calculating formal charge:
|
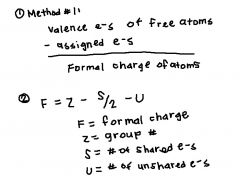
|
|
|
A summary of formal charge:
|

|
|
|
two important features of resonance structure:
|
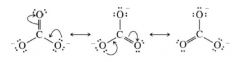
1. each atom has the noble gas configuration
2. we can convert one structure into any other by changing only the positions of the electrons. |
|
|
resonance theory:
|
whenever a molecule or ion can be represented by two or more lewis structures that differ only in the position of the electrons
1. none of the structures (resonance structures/ resonance contributors) 2. the actual molecule/ ion will be better represented by a hybrid (avg) of the structure. |
|
|
Electrostatic potential map:
|

- shows electron density
- regions of relativity more (-) charge are red - more (+) regions are indicated by colors trending toward blue. |
|
|
summary of rules for resonance:
|
1. resonance structures exist only on paper
2. in writing resonance structures we are only allowed to move electrons 3. all of the structures must be proper lewis structure 4. the energy of the actual molecule is lower than the energy that might be estimated for any contributing structure. 5. equivalent resonance structure make equal contributions to the hybrid, and a system described by them has a large resonance stabilization 6. the more stable a structure is (when taken by itself), the greater is its contribution to the hybride. a. the more covalent bonds a structure has the more stable it is. know that forming a covalent bond lowers the energy of atoms. b. structures in which all of the atoms have a complete valence shell of electrons (the noble gas structure) are especially stable and make large contributions to the hybrid c. charge separation decreases stability. d. resonance contributors with negative charge n highly electronegative atoms are more stable than ones with negative charge on less nonelectronegative atoms. |
|
|
wave functions:
|
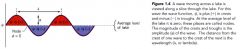
- most often denoted by the greek letter psi
- each corresponds to a different energy state for the electrons. ------ each state is a sublevel where one/two electrons can reside. |
|
|
orbitals:
|
is a region of space where the probability of finding an electron is large.
the greater the # of nodes, the greater the energy. |
|
|
Aufbau principle:
|
orbitals are filled so that those of lowest energy are filled first.
|
|
|
Pauli exclusion principle:
|
a maximum of two electrons may be placed in each orbital but only when the spins of the electron are paired.
- an electron is permitted only one or two other of just two possible spin orientations. either up or down spin. |
|
|
Hund's rule:
|
when we come to orbitals of equal energy (degenerate orbitals) such as the three p orbitals, we add one electron to each with their spins unpaired until each of the degenerate orbitals contains one electron. (this allows the electrons, which repel each other, to be farther apart). Then, we begin adding a second electron to each degenerate orbital so that the spins are paired.
|
|
|
example of electron configuration:
|

|
|
|
Bonding Molecular orbital:
|
1. contains both electrons in the lowest energy state (ground state), of a hydrogen molecule
2. formed when the atomic orbitals combine in the way by addition... atomic orbitals of the same phase sign overlap. a. leads to reinforcement of the wave function in the region between the two nuclei. 3. increasing the electron probability density in exactly the right place (region of space between nuclei) when the electron density is large, the attractive force of the nuclei for the electrons more than offsets the repulsive force acting between the two nuclei... "the glue that holds the atoms together" |

