![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
What is everything in the universe made out of?
|
Matter
|
|
|
What is mass?
|
Amount of matter in an object
|
|
|
What is volume?
|
Amount of space an object takes up
|
|
|
What do we use properties of matter for?
|
We use them to classify different kinds of matter
|
|
|
What is a property?
|
A property is any characteristic that identifies one substance from another
|
|
|
What are the basic properties of matter?
|
Mass
Weight Volume Density |
|
|
What are other properties of matter?
|
Color
Odor Ability to dissolve Boiling points |
|
|
What are elements?
|
Elements are the simplest pure substance
|
|
|
What cant elements do?
|
They cannot be changed into simpler substances by ordinary means
|
|
|
What are elements made out of?
|
They are made out of atoms
|
|
|
Is the atom of an element chlorine the same as an atom of the element sodium?
|
No
|
|
|
What is a compound?
|
A pure substance made of more than 1 element that has been chemically combined
|
|
|
What cant compounds do?
|
They cant be broken into simpler substances?
|
|
|
What is the smallest part of a compound?
|
Molecule
|
|
|
What is the classification of matter?
|
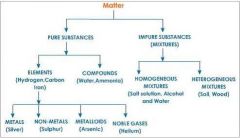
|
|
|
What do substances in a mixture do?
|
They keep their separate identities and most of their own identities
|
|
|
Can Mixtures be separated by physical means?
|
Yes
|
|
|
What is Heterogeneous?
|
"Least mixed" does not appear same throughout. Particles large enough to be seen to separate easily.
Ex: Chocolate chip cookies |
|
|
What is Homogenous?
|
"Well mixed"; same throughout particles are very small, not easily recognized and do not settle when allowed to stand
|
|
|
What is a Special Homogeneous Mixture (Solution)?
|
"Best mixed". A solution is a type of homogeneous mixture formed when 1 substance dissolves in another
|
|
|
What is a crystalline solid?
|
Particles arranged in regular, 3D,repeating pattern
Ex: Salt |
|
|
What is a amorphous solid?
|
Lacks a regular,repeating pattern
Ex: Glass and Wax |
|
|
What is "4th" phase?
|
Plasma: Hot gas of electricity charged particles. Particles actually shake violently at high temperature
Ex: In stars |
|
|
What are phase changes?
|
Phase changes are due to energy: More energy causes particles to move faster and farther apart
|
|
|
How do you change a phase change?
|
Adding or taking way energy
|
|
|
What = to freezing point?
|
Melting Point
|
|
|
What is sublimation?
|
The direct change from a solid to a gas without melting
Ex: Dry Ice |
|
|
What is the boiling point?
|
Temperature in which liquid boils at sea level
|
|
|
When does boiling occur?
|
Throughout the liquid
|
|
|
What does B.P also depend on?
|
Pressure
|
|
|
What is Evaporation?
|
Same but only occurs at the surface of the liquid
|
|
|
What is condensation?
|
Point that gas changes to a liquid
|
|
|
Are particles large enough to be seen?
|
No
|
|
|
Are particles evenly spread out?
|
Yes
|
|
|
What do substances do in a solution?
|
They retain their original properties just like a mixture
|
|
|
Are all solutions liquids?
|
No
Ex: Gold Jewelry- Gold + Copper |

