![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
127 Cards in this Set
- Front
- Back
|
what is the 'hallmark' characteristic of herpesviruses
|
the ability to achieve latency
– may be silent for months, years followed by reactivation – recrudescence. |
|
|
how do alpha, beta, and gammaherpesvirinae differ
|
Alphaherpesviruses grow rapidly, lyse cells and achieve latency in neural ganglia.
Betaherpesviruses are the cytomegaloviruses; replicate slowly, cell lysis later, achieve latency in secretory glands, lymphoid tissue, and other tissues. Gammaherpesviruses are lymphotropic; may be cytocidal in epithelial tissue; latency in lymphocytes; associated with oncogenesis. |
|
|
gammaherpesvirinae attack what cell type?
|
lymphocytes
|
|
|
Betaherpesvirinae acheive latency where?
|
secretory glands
lymphoid tissue other tissues |
|
|
Alphaherpesvirinae achieve latency where
|
in neural ganglia
|
|
|
Are herpesviruses associated with oncogenesis?
|
yes (gammaherpesvirinae)
|
|
|
herpesvirus
viral properties |
Icosahedral capsid.
- 162 capsomers, hollow. - 100-200nm Tegument around capsid - Fibrous, amorphous. - Contains viral enzymes involved in replication. Envelope around tegument – - Diameter ~120-300nm. - Derived from nuclear membrane. Core of linear dsDNA. - 120-230kbp - Repeat sequences at termini Capsid proteins – hollow. 8-12 envelope glycoproteins. - Designated gA, gB, gC……. - Function in attachment, fusion of envelope with cell membrane, penetration. - One binds Fc of IgG. Proteins involved in regulation and replication. Proteins involved in growth regulation and immunomodulation. Proteins also found in tegument. - alpha-TIF is an important activator of transcription - VHS – shuts down host protein synthesis |
|
|
know the appearance of the herpes capsid
|
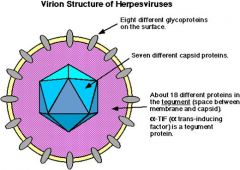
|
|
|
know the appearance of herpesviruses
|
|
|
|
know the appearance of herpesviruses
|
|
|
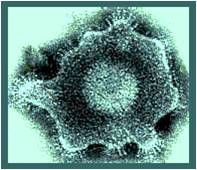
|
herpesvirus
|
|
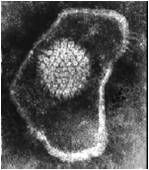
|
herpesvirus
|
|
|
herpesvirus genome
|
Inverted repeat sequences flank the genome.
Genes divided into 3 classes. alpha - immediate early - Regulatory proteins - Not packaged in virion beta - early - Replication enzymes incl DNA polymerase gamma - late - Structural proteins |
|
|
what is the purpose of the inverted repeat sequences associated with herpesvirus genome
|
Allow linear double stranded genome to fold back on itself
Repeat sequences sprinkled throughout the genome to help it fold up in different ways |
|
|
Herpesvirus attachment is mediated by ________
Fusion is mediated by ________ |
Attachment is always mediated by envelope glycoproteins.
Fusion is mediated by other peplomeres |
|
|
In herpesviruses _______ is like the mafia going in and taking out the competition
|
viral host shutoff protein
|
|
|
Herpesvirus
______ is important in starting the whole transcription process |
alpha TIF (trans-inducing factor)
Without it, transcription (1st step after uncoating) can’t happen |
|
|
herpesvirus replication - Complex
|
Attachment via viral envelope glycoproteins.
- Interacts with cell surface proteins, e.g. nerve growth factor receptors, TNF receptor. Fusion of envelope with cell membrane mediated by viral glycoproteins. Viral Host Shutoff (VHS) protein released from tegument; degrades host mRNA. Viral capsid with tegument proteins migrates to nuclear pore. Viral DNA “injected” into the nucleus, along with few viral proteins. - α TIF (trans-inducing factor) - Activates transcription of early proteins DNA replicated by rolling circle mechanism gamma genes activated. - Structural proteins made. - Include capsomers, envelope glycoproteins, and tegument. αTIF and VHS. Capsid is assembled in the nucleus. DNA genome packaged in preformed capsids by viral protein. - This protein also cleaves the concatemers. Capsid buds through the nuclear membrane, ER or plasma membrane. |
|
|
describe herpesvirus transcription
|
Transcription uses cellular RNA polymerase; viral proteins are also involved.
Early transcription divided into immediate and delayed phases. Products of immediate early (α) migrate back to the nucleus. Activate subsequent transcription; α4 important transcription activator – activate delayed early genes (β). Delayed early products mediate DNA replication. - Includes viral DNA polymerase. - Accumulation of these proteins leads to DNA replication. |
|
|
understand herpesvirus feedback system
|
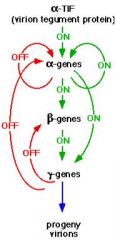
|
|
|
describe herpesvirus binding through penetration in general terms
|
Binding via envelope glycoproteins
Fusion via viral glycoproteins Migrates to nucleus Dumps genome and a few proteins into the nucleus |
|
|
understand how herpesvirus gets into the cell -> uncoating
|
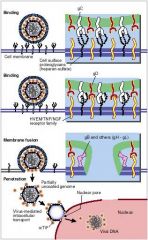
|
|
|
how does herpesvirus replication occur?
|
DNA is replicated by a rolling circle mechanism.
- Thymidine kinase – viral enzyme provides DNA precursors in nondividing cells, such as neurons. - Concatemers formed (multiple copies of the genome) This isn’t a circular genome (it’s linear) but it can circularize (due to repeat sequences) which helps make replication more efficient |
|
|
How does a herpesvirus get its envelope?
|
its envelope can come from different regions (golgi, nuclear membrane, ER, etc)
It can potentially bud through many membranes to get the envelope (not successive, it gets the envelope then loses it before it buds through the next membrane) |
|
|
herpesvirus
cellular effects |
Chromatin displaced, framework used by virus.
Cell membrane altered. - Env GP inserted, target for immune response. Intranuclear inclusions characteristic. Shut-down of cell metabolism. - DNA, RNA, protein synthesis, glycosylation all affected. - Leads to cell lysis. |
|
|
how are viral actions detrimental to the cell
|
Takes over synthetic machinery
Uses up the building blocks of DNA Envelope glycoproteins inserted into membrane in preparation for budding make the cell a target lytic infection |
|
|
describe herpesvirus latency
|
Virus enters nerve endings at site of replication, migrates to sensory ganglion.
Genome is maintained intact. Three phases: - Establishment - Maintenance - Reactivation Neuron infected acutely; most resolve; a few neurons remain infected with the herpesvirus genome episomal in the nucleus. Only 1 mRNA produced. - Latency associated transcript (LAT) - Unknown if protein produced from it; role unknown. Passively maintained – proteins of productive infection not produced. |
|
|
how does herpesvirus recrudescence occur
|
Reactivation (recrudescence) leads to productive viral replication at the initial site of infection.
Triggered by stress, other signals (immunosuppression) that lead to transcription in the neuron. Neuron must survive – how? - May be that only a few virions are made. - Viral proteins may inhibit death of the neuron. |
|
|
Herpesvirus
(viral integration during latency/ reactivation/ symptoms during recrudescence/ antibody production during latency) |
Viral integration during latency
- Episomal: Doesn’t integrate into the DNA, it just sits there until something triggers reactivation Reactivation - Forms a virus and moves down axon to replicate in the epithelial cells During recrudescent episodes, there may be no symptoms - So this time is a big concern No antibodies produced/ etc. while in latency |
|
|
common characteristics of herpesvirinae
|
Transmitted by close contact or droplet infection.
Persistent infection with intermittent shedding occurs in all; reactivation is associated with stress, and is usually subclinical. Alphaherpesviruses produce localized lesions in skin/mucosa; infection with many become generalized including to the fetus. - many are abortive Beta- and Gammaherpesviruses are not highly lytic; infections are chronic. |
|
|
1. _____ herpesviruses can be abortive
2. ______ herpesviruses are highly lytic 3. _______ herpesviruses are associated with oncogenesis 4. _______ herpesviruses achieve latency in lymphoid tissue. 5. ________ herpesviruses achieve latency in lymphocytes. |
1. alpha
2. alpha 3. gamma 4. beta 5. gamma |
|
|
1. ______ herpesviruses replicate slowly
2. _______ herpesviruses grow rapidly. 3. _______ herpesviruses achieve latency in neural ganglia 4. ______ herpesviruses achieve latency in lymphocytes 5. ________ herpesviruses achieve latency in secretory glands. 6. _________ herpesviruses may be cytocidal in epithelial tissue |
1. beta
2. alpha 3. alpha 4. gamma 5. beta 6. gamma |
|
|
which herpesvirus is lymphotropic
|
gamma
|
|
|
vetinary pathogens (herpesvirus)
|
Bovine – abortion, encephalitis, respiratory disease.
Equine – abortion, neurologic disease, respiratory disease. Swine – neurologic, reproductive and respiratory disease. Canine – “kennel cough”, neonatal death. Feline – respiratory disease. Wildlife species - Primates – Herpes simiae or Cercopithecine herpesvirus-1 of macaques is lethal to humans (“monkey B virus”). - Birds - Marine mammals - Elephants, Asian and African. - Probably occurs in most species. |
|
|
Bovine Herpesvirus-1
|
Alphaherpesvirinae
Infectious bovine rhinotracheitis (IBR). Respiratory disease, vulvovaginitis and balanoposthitis, conjunctivitis, abortion, enteritis, encephalitis (rarely). Aerosol, sexual, in utero transmission. Replicates in epithelial cells where entry occurs; also white blood cells - cell-associated viremia. - Like cooler temps so stay near resp tract but can spread systemic by hitching ride on WBC Balanoposthitis/Vulvovaginitis - Genital lesions (esp in dairy cattle) Respiratory disease with fever, nasal discharge, conjunctivitis; hyperemic mucosa (“rednose”), oral ulcers; predisposes to secondary bacterial infection of LRT. Diarrhea in neonatal calves. Abortion in pregnant cows several months after acute infection. |
|
|
BHV-2
|
Alpha
Bovine mammilitis – skin lesions on teats; “pseudocowpox”. Pseudolumpyskin disease – generalized skin nodules; resembles more serious poxvirus. - Most common in southern Africa. - Mechanical transmission by insects. |
|
|
BHV - 3, 4, & 5
|
BHV-5 – Alpha
- Encephalitis - Neural spread from nasal and oral cavities. BHV-3 and 4 – Gamma - Little clinical significance |
|
|
Alcelaphine Herpesvirus-1 & Ovine herpesvirus-2
|
Alcelaphine Herpesvirus-1 (wildebeest); Ovine herpesvirus-2 (sheep worldwide).
Malignant catarrhal fever. Fatal lympho-proliferative disease of cattle and some wild ruminants incl white tailed deer and bison. Affects lymphoid and epithelial tissue of respiratory, GI tracts, urinary tracts; vasculitis. Wildebeests are reservoirs in Africa; Sheep are thought to be reservoirs outside Africa. - Adapted hosts are asymptomatic - Disease in nonadapted hosts. Fever, depression, leukopenia, oculo/nasal discharge, extensive GI mucosal erosions, lymphadenopathy, CNS infection, corneal opacity. |
|
|
mad itch of swine is assoc with which virus
|
pseudorabies (alphaherpesvirus)
|
|
|
pseudorabies
|
Pseudorabies – Alphaherpesvirus
Swine are the reservoir, incl feral swine. Significant economically - USDA eradication efforts ongoing. Oral/intranasal exposure; neural and viremic spread; shed in saliva and respiratory secretions. Abortion/stillbirth/weak piglets in pregnant sows. Mortality in neonates. Respiratory disease in growing and mature swine with fever, depression, cough; in younger swine progresses to neurologic disease ending in death. In secondary hosts (cattle, dogs, cats, horses, sheep, wildlife), CNS involvement; aka “mad itch”; ends in death. Vaccination in endemic areas. |
|
|
what are the 2 porcine herpesviruses
|
pseudorabies (alpha)
Porcine cytomegalovirus (Beta) - Rhinitis in piglets – leads to weight loss and death. - May cause fetal death. - This one isn’t a biggie |
|
|
alphaherpesvirinae tend to attack what 4 tissue types
|
Epithelial cells
Resp tract Fetus Occ brain |
|
|
EHV 1&4
|
Alpha
Antigenically related. Respiratory transmission; viremia occurs. Rhinopneumonitis – with fever, nasal discharge; pneumonia in foals. Abortion in pregnant mares usually associated with EHV-1. Neural disease also associated with EHV-1 due to vasculitis. MLV and killed vaccines Can infect camelids |
|
|
EHV 2,3,&5
|
Equine Herpesvirus-3 – Alpha
- Equine coital exanthema - Pustular genital lesions - Mild disease Equine Herpesviruses-2 and - 5 – Gamma - Questionable significance |
|
|
most herpesviruses target what tissue type?
why? |
Most target resp epithelium
Like cooler temps - Impacts cellular tropism - Don’t usually spread systemically - Pneumonia rare, repro probs rare |
|
|
_____ is the only feline URT infection that affects the cornea.
|
feline herpesvirus-1 (alpha)
|
|
|
feline herpesvirus-1
|
alpha
rhinotracheitis/ also affects cornea Respiratory spread Targets epithelia of URT; prefers temp of 33-35C; Most severe in kittens. Sneezing, oculo/nasal discharge, salivation, anorexia; may have corneal lesions (keratitis). Very contagious; problem in shelters, catteries. Killed and MLV vaccines. - IgA and cell mediated immunity impt |
|
|
Canine Herpesvirus 1
|
Alpha
Mainly reproductive Danger time - Last 3 weeks of gestation and 1st 3 weeks of life Very little prob in adults Likes cool temps - Neonates have probs with regulating temp so can spread systemically in them Respiratory and genital spread. Prefers temp of 33-35C; pups have poor temp control. Associated with kennel cough in adults. Abortion, neonatal death most serious consequences. Pups infected in utero or oronasal. Cell-associated viremia Replicates in blood vessel walls. |
|
|
Avian Herpesvirus
Infectious Laryngotracheitis |
Alpha
hemorrhagic tracheitis potl for secondary pneumonia Gallid Herpesvirus-1 Transmitted by droplet inhalation. Necrosis and ulceration of URT; leads to asphyxia. Cough, sneezing, discharge, gasping, dyspnea. Mortality may be 50% or higher. Also associated with lower egg production. Vaccination in breeding flocks. |
|
|
Marek's Dz
|
alphaherpesvirus
Gallid Herpesvirus-2 Very important disease of poultry. Virus is shed in dander; inhaled. Replicates in respiratory epithelia followed by cell-associated viremia (macrophages). Targets lymphoid tissue; may lead to lymphocyte transformation (oncogenesis); these may infiltrate nerves. Asymmetric paralysis “grey eye” – infiltration of iris with neoplastic cells Skin nodules Healthy carriers occur. Vaccination in ovo |
|
|
duck plague
|
herpesvirus unassigned to subfamily/ genus
Anatid Herpesvirus 1 Domestic and wild ducks, geese, other waterfowl. Ingested; leads to enteritis. Viremia occurs with vasculitis; hemorrhages occur. Depression, ruffled feathers, tremors, diarrhea. Morbidity and mortality can be high as 100%. |
|
|
Psittacine herpesvirus
|
Wide variety of psittacines.
Pacheco’s disease. Shed in feces, nasal discharge. Liver is a major target. Lethargy, ruffled feathers, diarrhea, conjunctivitis, tremors. Significant mortality Vaccine available |
|
|
Primate Herpesviruses
|
B Virus of Macaques
- Cercopithecine Herpesvirus 1 - Transmitted through saliva. - Disease resembles HSV in humans. - Can be transmitted to humans via bite or scratch, or saliva contact with mucous membrane. - Ascending paralysis, encephalitis and death. - High mortality rate; survivors may have permanent impairment. - Assume all macaques are infected and treat accordingly in terms of safety issues. Simian Varicella - Cercopithecine Herpesviruses 6,7 and 9 - Old World monkeys. - Chickenpox-like signs |
|
|
fish herpesvirus
|
it is a significant pathogen with a high mortality
|
|
|
elephant herpesvirus
|
Lethal dz in captive elephants
Carditis and death in young elephants (high mortality) Originally in asian elephants (we thought) Turns out it was really from african elephants but they didn't have a problem with it |
|
|
herpesvirus epidemiology
|
Bovine – significant in feedlots, during shipping.
Porcine – Rodents important in PRV spread; significant outbreaks occur when weaned swine from several sources brought together. Others – situations of crowding, increased density predispose to spread. |
|
|
herpesvirus diagnosis
|
Virus isolation
Virus detection - immunofluorescence Serology - Many animals are seropositive. - Rising titer or IgM indicates primary infection. |
|
|
herpesvirus tx and control
|
Several antiviral drugs available for herpesvirus infections.
- Most are nucleoside analogs. - Activated in infected cells, lead to termination of DNA chain elongation. Vaccine available for many herpesviruses of domestic species. - BHV 1 - EHV 1 and 4 - PRV - FHV 1 - GHV 1 and 2, AHV 1 - Channel catfish virus Eradication - BHV-1 in some European countries - Pseudorabies |
|
|
how do antivirals help tx herpesvirus
|
adds a fake base
When that fake base is added, it ends the viruses ability to cause dz Only activated in infected cells Has a preference for polymerase of herpesvirus |
|
|
_______ is the largest animal virus
|
poxviridae
|
|
|
describe the morphology of poxviruses
|
Complex morphology
Dumbell-shaped core - Contains nucleic acid Two lateral bodies - Function unknown Pallisade layer – tubular to globular proteins. Lipoprotein membrane – core envelope (intracellular). Outer envelope derived from Golgi (extracellular). Loss of outer envelope does NOT remove infectivity. |
|
|
describe the poxvirus genome
|
dsDNA, 130-375 kbp (BIG!!); High A/T content
150-200 genes - Enzymes for replication. - Proteins that affect host immune response. Terminal hairpin loop - denature→ circle Inverted terminal repeats - Tandem repeats within these regions. Central region - Highly conserved - Genes for replication |
|
|
describe the ends of the poxvirus genome
|
Covalently linked at end
- If you denature it, it will circularize Ends are non-coding regions - Repeat sequences - Fxn in replication and some other regulatory capacity - Don’t encode proteins but they are impt |
|
|
why is poxviridae different than other viruses we have studied prior?
|
Everything is occurring in the cytoplasm
This is the 1st one that does this - Can’t utilize the cell enzymes - No splicing Everything is done with viral enzymes (still uses ribosomes) |
|
|
what are some properties of poxviruses
|
Fairly resistant
All vertebrate poxviruses share at least one antigen. - More extensive cross-reactivity within a genus. - Allows immunization with one species of virus within a genus to protect against another within the same genus. |
|
|
Poxviridae replication
|
Replicates entirely in cytoplasm.
- Steps occur in discrete areas - Viral factories - inclusions. Entry by fusion; may follow endocytosis. Core released. Virus encodes all the enzymes it needs for replication, incl DNA polymerase and RNA transcriptase. Early transcription commences upon entry. - Virus carries RNA polymerase as well as capping and poly A polymerase in mature virion. - Early transcription factor also carried in which insures early genes are transcribed first. - Early mRNA made from ~1/2 of genome. - No splicing (as virus rep in cytoplasm). - Products are primarily enzymes for DNA replication. DNA replicaton occurs a few hours after entry. - Mechanism not well-understood. - Several enzymes involved. - Generates concatemers. - Recombination may occur with other co-infecting poxviruses. - With replication of DNA, transcription shifts to intermediate and late genes. Product of early genes allows a shift to intermediate; one intermediate product is late transcription factors. Products of late transcription are mainly structural proteins. Some proteins packaged are transcription enzymes to be carried into next host cell. Virus assembles. Occurs in discrete sites – seen as inclusions. - Complex, requiring several hours. Release by budding through the golgi membrane (EEV) or through cell lysis (INV). |
|
|
what are the products of early, intermediate, and late transcription?
|
Early transcription
- Proteins not carried in that are needed for replication Intermediate and late - Structural proteins |
|
|
what are the steps involved in poxviridae infection?
|
1. Entry
2. Early transcription 3. Early translation 4. Modification of host response 5. Release of core 6. DNA replication 7. Separation of concatemers 8. Intermediate transcription 9. Intermediate translation 10. Late transcription 11. Late translation 12. Protein insertion in membrane 13. Assembly 14. “Wrapping” of core with cytoplasmic membrane 15. Release by lysis – lacks outer envelope 16. Budding – acquires outer envelope – virus now in Golgi. 17. Release by exocytosis 18. Cell-to-cell spread |
|
|
Poxvirus pathogenesis
|
Systemic or localized infection depending upon the virus.
Route of entry may be through abrasions or via respiratory tract (smallpox); some transmitted by arthropods. Replicates at site of entry in macrophages – may remain at site of entry. May enter lymphatics, and from there spread to bloodstream – systemic spread. Depending upon immune response and virulence, may see complications, such as toxemia, and death. |
|
|
poxvirus cytopathic changes
(how does it cause cell death) |
Cause cell death via a number of mechanisms.
Inhibit cell’s DNA, RNA and protein synthesis. - Via virus protein - Inhibition of initiation of cellular translation. - Viral messages > cell messages. - Host mRNA synthesis and transport inhibited. - Decreased half life of cell mRNA Alter membrane permeability. Uses up cell machinery |
|
|
how do some poxvirus viral proteins affect the host response
|
Defense against host response.
- IFN resistance - Block of complement cascade. - Modulation of inflammatory response. Some have growth promoting activity. - Viral Growth Factor - Binds Epidermal GF receptor to stimulate cell growth. - Increases viral yield |
|
|
orthopoxvirus
|
Smallpox (variola)
- Variola major mortality of ~25-35%. - Variola minor mortality of ~1% Vaccinia – vaccine virus. Monkey pox – rare zoonosis of Africa; can be fatal. Ectromelia – mouse pox Cowpox – rodent reservoir; also zoonotic. Others - Camelpox, buffalopox, raccoonpox…. |
|
|
_______ is the first disease against which a vaccine was developed
|
smallpox
|
|
|
______ was the first dz eradicated
|
smallpox
|
|
|
smallpox
|
Spread via respiratory tract (inhalation/ ingestion).
Spreads to regional lymph nodes then bloodstream. Enters epidermis causing necrosis evidenced as vesicles. - Similar lesion occur in internal organs. |
|
|
what was smallpox eradicable
|
Good cross-protection using vaccinia.
Stable vaccine. Easy to administer. No animal reservoir. No persistent/latent infection. Long-lasting immunity |
|
|
______ was the 1st virus grown in tissue culture
|
vaccinia
|
|
|
vaccinia
|
Descendant of cowpox or variola - unknown.
- Wide host range. - Induces good long-lasting immunity The vaccine is contraindicated in people who have or are: - Eczema, atopic dermatitis or other acute, chronic, or exfoliative skin conditions - Diseases or treatments which cause immunodeficiency or immunosuppression - Moderate or severe acute illnesses - Previous allergic reaction to smallpox vaccine or any of the vaccine components - Pregnancy - Breastfeeding mothers - Infants and children under 12 months of age |
|
|
orthopoxviruses
monkeypox |
Monkeypox - systemic
Acquired from infected primates. West and central Africa Zoonotic - Lab and zoo workers Similar to smallpox Pustular rash, fever, toxemia. Squirrel/rodent reservoir |
|
|
a lot of pox viruses have _____ hosts (doesn't cause dz in them)
|
rodent
|
|
|
cowpox
|
Cow is incidental host; host is rodents.
Localized infection in teats, udder; spread by milking. Also infects cats, humans, zoo animals (esp felids). - Serious in cats. May develop widespread skin lesions. - Secondary bacterial infection may occur. High mortality in cheetahs. Found in Europe, Russia |
|
|
Ectromelia
|
Problem in research labs.
Occurs worldwide due to inadvertent spread in lab mice and products. Infection through abrasions – systemic spread. May be rapidly fatal. - Necrosis of liver, spleen. - Death within hours. Chronic form exists. - Ulcerating lesions on feet, tail, snout. |
|
|
camelpox & buffalopox
|
Camelpox
- Severe generalized disease. - Important in Africa, Middle East, SW Asia - Case fatality rate may be 25%. Buffalopox - Similar to vaccinia. - Water buffalo in Egypt, Indian subcontinent, Indonesia. - Pustular lesions on teats and udder; occasional generalized disease in calves. |
|
|
parapoxviruses
|
Cause localized lesions.
Can be economically significant. Zoonotic – papular lesions. |
|
|
Orf
|
Parapoxvirus
sheep, goats Contagious pustular dermatitis. lesions primarily in mouth, muzzle, lips. May prevent lambs from nursing – economically significant. Spread by direct contact, fomites. Vaccine available – ewes near lambing. Orf is zoonotic - It’s not forever but it’s not fun |
|
|
parapoxviruses
pseudocowpox/ papular stomatitis |
Pseudocowpox
- Common in cattle worldwide. - Lesions on teats, muzzle. Papular stomatitis - Worldwide in cattle. - Lesions on muzzle, lips. |
|
|
Capripoxviruses
|
Lumpy skin disease.
Cattle – Bos taurus, and Bos indicus. Africa – spread throughout continent. Fever, nodular lesions in skin, viscera – systemic disease. Nasal and ocular discharge, anorexia; ulcerated skin lesions. High morbidity, mortality usually low. Prolonged convalescence. Transmitted by insects. Wildlife reservoir. Economically significant – decreased productivity, damage to hides Controlled by vaccination where endemic. Fever, nasal discharge, anorexia – systemic dz. Cutaneous nodules develop; become secondarily infected. Visceral lesions, incl. lung. Spread by insects, fomites, respiratory droplets. |
|
|
systemic poxviruses
(suipox/leporipox) |
Suipox
- Swinepox; - Mild disease, skin lesions. - Seen sporadically worldwide. - Transmitted by biting louse. Leporipox - Myxoma – rabbits; - High mortality in European rabbits with generalized disease. - Localized benign fibroma in rabbits of Americas. - Transmitted via insects. - Shope fibroma. - Squirrel fibroma. - Hare fibroma. |
|
|
Avipox
|
Fowlpox – poultry.
Chickens, turkeys. Vaccines available. Spread by biting insects. - Papules on comb, wattle, around beak. - Lesions may occur on legs, feet. - Heal in 2-3 weeks. Droplet infection may occur. - Mucous membranes of oral cavity, pharynx, trachea. - Lesions coalesce – can asphyxiate. - Poor prognosis. |
|
|
most avian poxviruses are seen where?
|
Usually see on feet
Hard to perch (big prob in passerines) Can make difficult to eat |
|
|
dx of poxviruses
|
Classic lesions
Histopathology - inclusions Virus detection - Virus isolation - Electronmicroscopy - Polymerase chain reaction (PCR) Serology |
|
|
poxviridae tx and control.
|
Immunoglobulin
Supportive Recovery leads to life-long immunity. Vaccine - Veterinary vaccines in use for many poxviruses. - Smallpox vaccination is beginning to be implemented again due to concern over bioterrorism. - Primarily in “front line” individuals – health care workers, military, law enforcement. |
|
|
poxviruses as vectors
|
Promotes recombination
Live vaccine - b/c virus producing protein, get presentation in MHC1 |
|
|
_____ is an impt virus in commercial fish production
|
iridovirus
|
|
|
african swine fever is what kind of virus?
|
asfarvirsu
|
|
|
asfarviridae/ iridoviridae properties
|
Asfarviruses
- enveloped, 175-215nm in diameter. - Complex icosahedral capsid. - Genome of linear dsDNA 170-190kbp. - Covalently closed ends. - Inverted terminal repeats. - Encodes up to 200 proteins. Iridoviruses - Morphologically similar to asfarviruses. - Enveloped, 160-200nm. - Icosahedral capsid. - Genome 95-190kbp. |
|
|
where to asfar/iridoviridae replicate?
what is required for their replication? |
Will not replicate in anucleate cells
Asfar - Replicates in cytoplasm, but needs a nucleus for some unknown reason Irido requires a nucleus (b/c uses cellular transcriptase) - Transcription occurs in nucleus - Replication/ assembly in cytoplasm |
|
|
describe asfarvirus replication
|
Replication occurs primarily in the cytoplasm.
Nucleus is needed for DNA synthesis. Virus provides enzymes for transcription and replication. After entry, virus uncoats, begins transcription with viral enzyme. DNA replication similar to poxviruses, producing concatemers. |
|
|
how does iridovirus replication differ from asfarvirus
|
Use cellular RNA polymerase for transcription modified by viral proteins to favor viral mRNA synthesis.
Initial replication is in nucleus; later in cytoplasm. |
|
|
African Swine Fever
|
Asfarvirus
ticks are reservoir - pigs are accidental host (so they get sick) Infects domestic swine, and other suids. Infection may be acquired via the respiratory tract, direct contact, or via tick bite. - Transtadial, transovarial transmission in ticks. - Pigs accidental host. Replicates in leukocytes, first locally in lymphoid tissue, then disseminates via the blood. - Initially, monocytes and macrophages. - Leukopenia results. - Also severe thrombocytopenia. - Virus inhibits secretion and synthesis of antiviral and proinflammatory cytokines in infected macrophages. - Virus disseminates widely to RES - widespread hemorrhages Many tissues infected. Swine that survive remain persistently infected. Virus is shed in all secretions/excretions. Neutralizing antibodies not produced. - Unknown why. - Obstruction to vaccine development. After incubation of 1-2 weeks, swine develop high fever. After onset of the fever, the animals stop eating and develop diarrhea, incoordination, and prostration. Some develop dyspnea, reddening or cyanosis of the ears, and hemorrhages. Mortality may be 100%. Adult warthogs do not develop any signs of disease. - Able to replicate |
|
|
how do you dx african swine fever
|
Signs and lesions.
- Febrile disease associated with hemorrhage and death. - Important to distinguish from hog cholera. Virus isolation and antigen detection on tissue and blood. - Immunofluorescence most common. - Notifiable! |
|
|
epidemiology of african swine fever
|
(asfarvirus)
Sylvatic cycle in warthogs in Africa. - Asymptomatic infection in wild pigs; develop viremia. - Argasid ticks which live in pig burrows are biologic vectors. Epidemic/endemic cycles in domestic swine. - Outbreaks in domestic swine can be via ticks or consumption of contaminated food (e.g. uncooked meat from infected swine). - Ticks in other regions of the world can transmit if virus enters the region. - After introduction, disease spreads by aerosol primarily; also indirect spread. Currently endemic only in sub-Saharan Africa and Sardinia. |
|
|
why is african swine fever a serious threat to swine industries throughout the world
|
No vaccine.
Transmission in meat products. Persistent infections. Tick vectors. |
|
|
what virus is associated with mass die-offs in frogs?
|
ranavirus (iridoviridae)
|
|
|
_______ is second only to parvo in terms of hardiness in the environment
|
caliciviridae
|
|
|
Caliciviridae classification
|
Vesivirus
- Vesicular diseases of cats, cetaceans, primates, skunks, reptiles. Lagovirus - Rabbit hemorrhagic disease. Norovirus - Norwalk virus. Sapovirus - Sapporovirus. |
|
|
Calicivirus properties
|
Icosahedral capsid.
- Nonenveloped. - small ~25-40nm - 32 cup-shaped capsomers. - Hardy in environment. ssRNA genome. - Linear - Positive polarity. - 7.4-7.7 kb - 5’ VPg protein – acts like a cap; - 3’ polyA tail. - Resistant to heat and detergent. |
|
|
how does the calicivirus genome differ from the picornavirus genome?
|
Notice that the structural proteins are at the 3’ end
of the ORF (vs Picornaviruses 5’ orientation) |
|
|
calicivirus replication
|
Similar to picornaviruses.
Occurs in cytoplasm. After entry and uncoating, viral genome is translated into a polyprotein that then undergoes cleavage. One product is viral RNA polymerase. Carries out transcription of genome. Some caliciviruses produce subgenomic mRNA (feline, porcine). Assembly of capsomers, packaging of genome, released by cell lysis. |
|
|
norwalk virus
|
calicivirus
Fecal-oral spread. Gastroenteritis – diarrhea, vomiting lasting 24-48 hours. “Cruise-ship virus” Worldwide; 23 million cases in US every year. Diagnosed by identification of virus in feces or serology. |
|
|
calicivirus veterinary pathogens
|
Feline calicivirus – upper respiratory tract infection.
- Newer syndrome associated with vasculitis. Vesicular exanthema of swine – eradicated in US as it resembles FMD; occurs also in marine mammals. Rabbit hemorrhagic disease – lethal infection, high morbidity and mortality; massive hemorrhages. San Miguel sea lion virus |
|
|
vesicular exanthema of swine
|
Present in marine mammals (they are the host).
- only animal affected is swine can enter though skin but normally oro-nasal exposure Indistinguishable from FMD – eradicated from US by 1956, not known to occur elsewhere. Systemic infection. Acute febrile disease with vesicle formation on snout, tongue, teats, oral cavity, and feet. Most infections occurred from contaminated feed. When occurred, was also associated with diarrhea, encephalitis, myocarditis, and abortion. Many antigenic serotypes. Notifiable. |
|
|
san miguel sea lion virus
|
calicivirus
Same as VE of swine. - Source of disease for swine. Abortion and vesicular lesions in sea lions on San Miguel Island. Carcasses fed to swine transmit virus. 17 types found in variety of sea mammals. |
|
|
rabbit hemorrhagic dz
|
calicivirus
Identified in China in 1984. Killed nearly half a million rabbits in 1st 6 months. Hemorrhagic lesions in lungs and liver. Spread through Europe by 1988 to N. Africa. Has been found in North America, but only European rabbit susceptible to disease. high mutation rate b/c RNA so it will probably not work to eradicate rabbits in australia Highly infectious disease of European rabbits. Disseminates to liver after entry (ingestion – fecal-oral) leading to necrosis. Leads to disseminated intravascular coagulation (DIC) and hemorrhages. Disease in rabbits >2 months of age (?). - affects older rabbits more Depression, fever, death. Hemorrhages and clots seen post mortem. Mortality 90%; morbidity virtually 100%. Use for biocontrol of rabbits in Australia. Vaccine available. |
|
|
feline calicivirus
|
One of two major causes of viral respiratory disease in cats.
Strain variation – impacts diagnostics and vaccines. Transmitted via fomites, aerosol - contagious. Lesions primarily in RT: - Esp URT Replicates in epithelia. Conjunctivitis, rhinitis. Oral ulcers. Pneumonia with some strains. Mortality highest in kittens. Don’t normally see corneal ulcers in these cats. Some strains very pneumotropic (profound pneumonia, causing death in 48 hours) Usually fairly mild New virulent systemic strains - Hemorrhagic strains noted in recent years. - Vasculitis. - Edema, crusting cutaneous lesions, hepatic involvement. - Unclear pathogenesis. - High mortality. Limping kitten syndrome – immune-mediated. After recovery, affected cats may shed the virus for months or longer from oropharyngeal region. This is a concern in hoarders |
|
|
calicivirus dx, tx, control
|
Diagnosis
- Virus detection - Serology No specific treatment – supportive only. Control. - Vaccine for feline calicivirus. - No vaccine developed for Norwalk nor Hepatitis E virus |
|
|
hepevirus
|
Genus without a family….
- only member is Hepatitis E - Small (25-35 nm) nonenveloped icosahedral virus. - Genome ~7000 bases in length. - 5’ cap - 3’ poly-A tail - very similar to calicivirus Replication in cytoplasm After uncoating, first ORF is translated – Viral RdRp. RdRp copies pos genome → neg genome: From the negative strand, genome length and subgenomic (ORF 2 and 3) length mRNA are made. Structural and nonstructural proteins made. Virus is assembled. Virus is released by cell lysis. |
|
|
Hepatitis E
|
Pathogenesis similar to hepatitis A virus.
- Ingested, spreads to liver. - Jaundice, malaise, nausea, abdominal pain. - No persistent infection. - Most recover. - Disease seen mainly in 15-40-year-olds. Another infectious hepatitis Shed in feces Sticks around in environment for a long period of time Affects those in the prime of their life Most significant in pregnant women (high mortality rate) - Esp in 3rd world countries Targets primarily 15-40 yo’s; ill for several weeks - ~2% mortality. ~20% mortality in pregnant women – fulminant hepatic failure. Four genotypes. - 1 and 2 humans only - 3 and 4 infects humans, pigs, other species. Rodents may be the reservoir. Similar virus identified in birds. |
|
|
astrovirus
|
Isolated from many mammalian and avian species
Icosahedral 28-35nm nonenveloped. Replication, structure, pathogenesis similar to norovirus - Gastroenteritis. - Many serotypes Star liked appearance - Calici/ hepatitis E/ astro are really hard to tell apart No envelope so hardy in environment Replication similar to calici Causes gastroenteritis like norovirus |
|
|
what are the 2 major diseases associated with picornaviridae?
|
polio
foot & mouth dz |
|
|
Picornaviridae classification
|
Enterovirus
- Enteric viruses - Poliovirus - Other diseases in veterinary medicine. - Swine vesicular disease. - Enteroviruses of cattle, swine, birds, monkeys. Rhinovirus - Virus of the common cold. - Also occur in cattle. Hepatovirus - Hepatitis A virus - Avian encephalomyelitis Aphthovirus - Foot and mouth disease virus. - Other veterinary pathogens, incl equine rhinovirus-1. Cardiovirus - Encephalomyocarditis of rodents. - Theiler’s murine encephalomyelitis virus. |
|
|
Picornaviridae Properties
|
Icosahedral capsid
- Nonenveloped - 22-30nm (small!) - 60 capsomers; 4 polypeptides, VP1-4. - “canyons” between virus proteins in capsid important for interaction with cell receptor. - Immunity to one doesn’t give immunity to another - Hardy in environment Genera vary in acid stability – influences tropism. - Aphtho unstable below pH7. - Rhino unstable below pH5 - Others stable at pH3. ssRNA genome Positive polarity ~7.5kb One ORF – 3 regions - 5’-most: Capsid proteins - 3’-most: Nonstructural proteins incl protease, RNA pol 3’ Poly-A tail, protein at 5’ end (VPg). 5’ non-translated region highly conserved. - Important in replication - Internal ribosomal entry. |
|
|
picornavirus replication occurs where?
|
replication in cytoplasm
doesn't need nuclear fxn |
|
|
picornavirus replication
|
Attachment to cell receptor.
- Members of immunoglobulin superfamily. - Integrins, other glycoproteins. Replication occurs in the cytoplasm of the cell. - Forms pore in cell membrane after attachment, injects nucleic acid. Genome replication. - RNA synthesis primed by VPg. - Viral and cellular proteins involved. no early and late translation Viral RNA dependent RNA polymerase transcribes genome. Full length negative strand made (using positive strand as template); this serves as template for more positive strands. Early in infection, + strands serves as mRNA; later, packaged in mature virion. Polypeptides assemble into capsomer (1). Capsomers assemble into capsid (2). Nucleic acid packaged (3). Virions released by cell lysis. |
|
|
how does picornavirus ensure that viral genome is preferentially translated.
|
Host translation interference.
- Cap-binding complex cleaved by viral protease. - Cellular mRNA uncapped. Viral message doesn’t need cap. - Internal ribosomal entry site. - Genome has 3’-poly A tail. - Single ORF – polyprotein cleaved post-translationally. 5’-UTR of the RNA folds into a cloverleaf, binds cellular proteins initiating translation |
|
|
picornavirus cellular effects
|
cytopathic effects
ds RNA accumulates (during replication it is ds, but the final product is ss) – stimulates IFN. RNA synthesis of cells inhibited by viral protein. Toxic effects of coat proteins. Altered ionic environment due to altered plasma membrane permeability. Cellular translation interfered with. - Viral mRNA out-competes host mRNA. - Inactivation of translation factors. |
|
|
enteroviruses
|
picornaviridae
Attacks from the intestine (from the lumen of the intestine) - Attacks epithelial cells and lymphoid tissue in intestine Occur in most vertebrates. Fecal-oral spread. Survives transit through stomach Replicates in intestinal lymphoid tissue. Stable at pH of 3 May spread systemically via bloodstream or neural infection. Important human pathogen. - Polio virus - Enteric disease - Respiratory disease - Myocarditis - Meningitis - Rash |

