![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
125 Cards in this Set
- Front
- Back
|
6 Types of intermediate filaments
|
1. Keratins - epithelial, hair, nails
2. Desmin - muscle cells 3. Vimentin - embryo, fibroblasts, leukocytes, endothelial cells 4. Glial Fibrillary Acidic Protein - astrocytes, Schwann Cells, oligodendrocytes 5. Neurofilaments - neurons 6. Nuclear Lamins - nucleus of all cells |
|
|
Drugs that act on actin
|
Cytochalasin ABCDE - secreted by fungi; bind to ends of actin filaments and prevent further polymerization
Phalloidin - from mushroom; binds to actin filaments and stabalizes them against depolymerization |
|
|
Molecules required for actin polymerization
|
Ca 2+, ATP, G-Actin
|
|
|
Functions/Types of Actin Bundles
|
1. Contractile: ex. cleavage furrow (myosin)
2. Gel-like network: ex. cell cortex (filamin) 3. Parallel: ex. core of microvilli Ivillin, fimbrin) 4. Focal Contact for Cell Attachment: ex. stress fiber (alpha-actin, talin, vinculin) |
|
|
5 functions of Intermediate Filaments
|
1. structural support
2. deformable 3D framework 3. anchor nucleus 4. adaptable connection between cell membrane and cytoskeleton 5. framework for maintenance and reorganization of nuclear envelope |
|
|
What are the "bones and muscels" of the cell
|
Intermediate filaments
They have no contractility and no polarity Mainly a crosslinking function |
|
|
What molecules are required for microtubule assembly
|
Mg 2+, GTP, MAPs
|
|
|
Tubulin Binding Drugs
|
Colchicine, Colcemid: inhibit addition of tubulin to microtubules
Vinblastine, Vincristine: cause tubulin aggregates to form Taxol: Stabalizes microtubules |
|
|
What is the function of MAPs
|
Organize microtubules and affect their stability
|
|
|
Describe the structure of the nuclear pore
|
80-100nm
3 rings - cytoplasmic, middle, and nucleoplasmic 8 spoke like structures that connect cyto. and middle rings nuc. ring has protruding basket middle ring contains transporter |
|
|
Where are ribosomes produced
|
Nucleolus
|
|
|
Fibrillar center
|
inactive DNA in nucleolus
|
|
|
Pars Fibrosa
|
RNA in nucleolus
|
|
|
Pars granulosa
|
maturing ribosomal subunits in nucleolus
|
|
|
nucleolar matrix
|
involved in nucleolar organization
|
|
|
A. Kinetochore
B. Kinetochore microtubule C. Astral microtubule D. Polar microtubules E. Chromosome F. Pole, centrosome G. Spindle H. Aster I. Zone of Interdigitation |
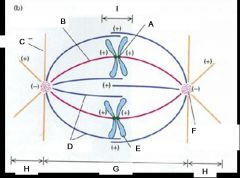
Name the structures
|
|
|
Describe how chromosomes seperate during anaphase
|
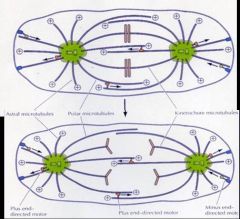
It's the assembly and disassembly of microtubules that allows chromosomes to seperate
|
|
|
What are nucleoporins and what are the two types
|
Nucleoporins are the proteins that line the nuclear pore complex and are responsible for the transport of all macromolecules. Importins transport molecules into the cell and exportins transport molecules out of the cell.
|
|
|
What is Kinesin
|
One of the motor proteins responsible for chromosome movement
|
|
|
What are three common types of membrane lipids
|
1. Phospholipids
2. Glycolipids 3. Cholesterol |
|
|
What is the general structure of a phospholipid and why is it amphipathic
|
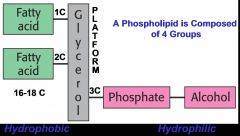
The fatty acid side chains can be the same or different and the alcohol is not always present
|
|
|
What is the general structure of a glycolipid and why is it amphipathic
|
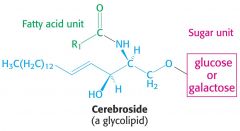
Similar to a phospholipid, a sugar takes the place of the phosphate group. Fatty acid tails are hydrophobic and sugar is hyfrophilic
|
|
|
What is the general structure of cholesterol and why is it amphipathic
|
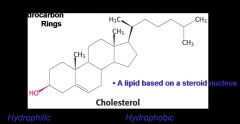
The hydroxol group is hydrophilic while the rest of the molecule is hydrophobic
|
|
|
What are 6 functions of the plasma membrane
|
1. Regulate nutrient and ion transport into the cell
2. Regulate transport of waste out of the cell 3. Maintain correct chemical conditions in the cell 4. Provide a site for lipid based chemical reactions 5. Interact with other cells or the ECM (extra cellular matrix) 6. Detect and transduce signals from environment to cell |
|
|
What type of molecules can pass directly through the lipid core of the membrane and which cannot
|
Can: Steroid hormones, Gases, small uncharged polar molecules, water and urea depending on the concentration gradient
Cannot: Large uncharge polar molecules, ions, charged polar molecules |
|
|
What are the features of a peripheral membrane protein
|
loosely associated with membrane
Do no enter or span the lipid core removed with mild conditions (salt/pH) |
|
|
What are the features of an integral membrane protein
|
Tightly associated with the membrane, enetering or spanning the lipid core
Removed only with harsh conditions (detergents) Contains one more stretch of 20-25 hydrophobic residues arranged in an alpha helix to span the lipid core |
|
|
Why is the hydrophobic alpha helix perfectly suited to span the lipid core
|
It has hydrophobic residues
All of the hydrogen bonds are intramolecular and are therefore not available to interact with the hydrophobic core |
|
|
What are the four types of lipid anchors
|
1. Farnesylation
2. Palmitoylation 3. Myristoylation 4. Glycosylphosphatidylinositol (GPI) Anchor |
|
|
How do lipid anchors work
|
Hydrophobic molecules that are covalently linked to a protein. The hydrophobic molecule is embedded in the hydrophobic core of the plasma membrane, thus anchoring the protein at the membrane. Importatn for recognition and signaling proteins.
|
|
|
How does cholesterol affect membrane fluidity with relation to Tm (phase transition or melting temp.)
|
Below Tm: Increases fluidity, kinked structure disrupts the tight and orderly packing of the lipid side chains
Above Tm: Decrease fluidity, limits the free movement of the lipid side chains due to its planar shape |
|
|
5 Reasons membrane fluidity is important
|
1. Influences arrangement of of proteins and lipids
2. Foster assembly/disassembly of protein subunits and signaling complexes int he membrane 3. Changes membrane permeability 4. Excessive fluidity can lead to membrane destruction 5. Altering fluidity can alter membrane and/or cell function |
|
|
How does fatty acid saturation affect membrane fluidity
|
Desaturated fatty acids have more kinks which disrupts tight packing thereby increasing fluidity and lowering the Tm compared to saturated fatty acids
|
|
|
How does the length of the fatty acid chain affect membrane fluidity
|
Longer acyl side chains increase the Tm
|
|
|
What are the four basic tissue types
|
1. Epithelial
2. Connective Tissue 3. Muscle 4. Nerve |
|
|
What are the 4 types of junctions of the epithelial lateral membrane
|
Zonulae Occludentes
Zonulae Adherentes Maculae Adherentes (Desmosomes) Gap Junctions |
|
|
Zonulae Occludentes
|
Tight junctions, most apically located, prevent movement of membrane proteins from apical to basolateral domain, fuse plasma membranes of adjacent cells to prevent water-soluble molecules from flowing in between, can be tight or leaky
|
|
|
Zonulae Adherentes
|
belt like junctions that adhere adjoining epithelial cells to each other, located just basal to the zonulae occludentes, the integral protein cadherin fills intercellular space and bind a bundle of actin filaments inside cell by alpha actinin and vinculin. Extra cellular regions of cadherins from adjoing cells bind to each other. This junction not only links the cell membranes of two adjoining cells but also thier cytoskeletons
|
|
|
Maculae Adherentes
|
Desmosomes - weld like junctions along the lateral cell membranes of epithelial cells that help to resis shearing forces. Attachment plaques of adjoing cells are located on the cytoplasmic side of the membrane. Intermediate filaments insert into plaque and extend into cytoplasm. The intercellular region is filled with the extra cellular region of a cadherin type transmembrane linker protein that binds the two cells in the presence of Ca 2+
|
|
|
Gap Junctions
|
Nexus or communication junctions: mediate intercellular communication by permitting the passage of various substances between adjacent cells.
^ molecules of the transmembrane protein connexin form a connexon - an aqueous pore through the plasma membrane to the intercellular space Connexons from adjoing cells fuse |
|
|
3 specializations of the basal surface of epithelial cells
|
1. Basal Lamina
2. plasma membrane enfoldings 3. hemidesmosomes |
|
|
What is the structure and function of hemidesmosomes in epithelial cells
|
Attach the basal cell membrane to the underlying basal lamina. An attachment plaque composed of desmoplakins is presenton the cytoplasmic side of the membrane. The cytoplasmic side of the transmembrane linker proteins of the integrin family attach to the plaque while their extracellular part bind to laminin and type IV collagen of the basal lamina. Keratin tonofilaments insert into the plaques on the cytoplasmic side
|
|
|
What are the main differences between exocrine and endocrine glands
|
Exocrine glands secrete their products via ducts onto the epithelial surface from which they originated.
Endocrine glands have lost their connection to their originating epithelium and secrete their products into the blood or lymph |
|
|
List and describe the four modes of exocrine secretion
|
1. holocrine: the entire cell disintigrates and releases its product (sebaceous gland)
2. Merocrine: exocytosis, secretory vesicle fuses with membrane and realeases content w/o loss of cytoplasm, most common (sweat, salivary, pancreas, goblet cells) 3. Apocrine: secretion is pinched off cell with some of cytoplasm n cell membrane (lipid portion of milk of mammary gland) 4. Cytocrine: whole cell is secreted (ovary and testis) 2. Merocrine |
|
|
What are the three parts of connective tissue
|
cells, ground substance, and fibers
|
|
|
What is the function of connection tisue
|
connects all the other cells of different tissue types both mechanically and medtaboloically, provides and maintians form of body
|
|
|
What germ layer does connective tissue derive from
|
mesoderm
|
|
|
3 classes of connective tissue
|
embryonic, proper, special
|
|
|
embryonic connective tissue
|
mesenchyme
mucoid connective tissue (umbilical cord) |
|
|
Connective tissue proper (4 types)
|
Areolar - loose packing and surrounds blood vessels
Dense connective tissue - 1. dense irregular (dermis, organ, capsules, periosteum), 2. dense regular (tendons, ligaments), elastic (ligamentum nuchae of the neck and flava) reticular tissue - lymphatic tissue, bone marrow; delicate network of reticular fibers adipose tissue - subcutaneous n omentum |
|
|
Special connective tissue (3 types)
|
cartlidge, bone, blood
|
|
|
7 Functions of onnective tissue
|
1. mechanical support - structural support to organs, packing material (nature's mortar), rigid structural support ( cartilidge and bone)
2. mechanical protection - bony and cusion 3. energy storage and temp regulation (fat) 4. metabolic support - regulates exchange of metabolites between blood and tissue 5. transport of material - blood and lymphatics 6. protection against infection - immune system works in CT 7. repair after injury |
|
|
stroma (def.)
|
general term for connective tissue of an organ
|
|
|
Parenchyma (def.)
|
general term for functional part of organ
|
|
|
3 classes of cells of connective tissue
|
fixed, wandering, associated
|
|
|
5 types of fixed cells of connective tissue
|
fibrocytes, mesenchymal, reticular, adipose, brown adipocytes
responsible for the synthesis and maintenance of the extracellular maintenance |
|
|
4 tyes of wandering cells
|
macrophages, mast cells, plasma cells. leukocytes
transient cells that migrate into connective tissue |
|
|
3 types of associated cells
|
endothelial, smooth muscle, pericytes
cells associated with blood vessels that are always within CT |
|
|
Fibrocytes
|
Fixed cell of the CT
Most common cell in CT proper Appearance - vesicular nucleus w/nucleolous, can't see cytoplasm, hard to identify Secretory cell - stuff of extracellular matrix fibroblast is still dividing and laying down new matrix, fibroblasts do not divide and only maintain existing matrix |
|
|
Mesenchymal cell
|
cell of embryonc CT, looks like fibroblast and has same function but only present in embryonic tissue
Pluripotential |
|
|
Reticular Cell
|
Found in Retucular CT
Has processes that form around reticula fibers forms cell covered fiber framework Secretory - matrix proteins |
|
|
Adispose Cells
|
Found in CT
2 types - white and brown white Morphology - large cell, signet ring, unilocular, forms in subcutaneous fascia white fuction - lipid storage white pathology - hypercellular obesity (increased # of adipose cells) and hypertrophic obesity (increased vol. of adipose cells) brown morphology - small, multilocular, many mitochondria brown function - provides heat by uncoupling oxidation process from ATP production (THERMOGENIN) brown location - newborns: mediastinum, aorta, axilla between scapula |
|
|
Lipid storage enzymes
|
lipoprotein lipase - breaks down lipids into free fatty acids and glycerols for storage as triglyceride
hormone sensitive lipase - breaks down triglycerides into free fatty acids for circulation |
|
|
Macrophages
|
Wandering cell of CT
Primary functions are phagocytosis and antigen presentation Lots of lysosomes and phagolysosomes ID w/phagocytosis of vital dye Part of mononuclear phagocyte system which develop from Granulocytic-Monocyte Stem Cell *When a monocyte leaves the blood for the CT, it becomes a macrophage, name depends on where it is |
|
|
Mast Cell
|
Wandering cell of CT
Morphology - very granular, hard to see nucleus Granules - mediators of inflammatory respone in response to IgE (Paracrine secretion) Location - along capillaries in mucosal membranes in resp, digestive, and urinary tracts |
|
|
What is in the granules of Mast Cells
|
histamine, heparin, eosinphilic chemotaxic factor, neutrophilic chemotaxic factor, leukotrienes, cytokines
|
|
|
Anaphylactic Shock
|
Too much release of Mast cell granules causes extreme sytemic inflamation and can couse death within hours due to migration of fluid into CT which causes bp to drop and smooth muscle contraction (lungs)
|
|
|
Plasma Cell
|
Wandering cell of CT
Morphology - nucleus is located to one side (eccentric) w/ cartwheel appearance of heterochromatin, many RER (to produce IgG) Produced from B-Cells and only found in CT under normal conditions |
|
|
3 types of extracellular fiber
|
collagen fibers, reticular fibers, elastic fibers
|
|
|
Type I Collagen
|
Strength
Dermis, tendon, organ, capsule, bone, dentin (most common) |
|
|
Type II Collagen
|
resists pressure
hyaline and elastic cartlidge |
|
|
Type III Collagen
|
delicate support meshwork
lymphatic system, spleen, liver, lung, cardiovascular system |
|
|
Type IV Collagen
|
Attachment, filtration
Basal Lamina |
|
|
Scurvy
|
Collagen disease caused by lack of vittamin C which is required for hydroxylation of proline
|
|
|
Ehlers-Danlos, Type VII
|
Collagen disease due to procollagen peptidase change
hyperflexible joints, dislocations, soft skin |
|
|
Ehlers-Danlos, Type IV
|
Due a deficiency in Type III Collagen
Aneurysms and intestinal rupture common |
|
|
Emphysema
|
Elastin fibers in alveoli force air out. Emphysema is a lung disfunction caused by the breakdown of elastic fibers in the alveoli
|
|
|
Marfan's Syndrome
|
Caused by poor microfibril formation in elastic fibers. Tendency to rupture aorta and other blood vessels
|
|
|
3 components of ground substance and thier functions
|
proteoglycans - viscosity, slippery
interstitial fluid - disspersion of nutrients and waste glycoproteins - adhesion molcules |
|
|
Types of GAGs
|
non sulfated - hyaluronic acid
sulfated - keratan sulfate (cartilage), heparin sulfate (basement membrane), chondroitin-6-sulfate (cartilage, bone, skin), Chondroitin-4-sulfate (artilage, bone, skin), dermatin sulfate (dermis) |
|
|
Proteoglycans
|
Protein core with sulfated glycosamines (GAGs) attached, bottle brush appearance
Pull water into ground substance |
|
|
Hyaluronic Acid
|
non sufated, long chain, neg charged, atracks cations and pulls in water, slippery
Has gel-like consisency that resists compression forms a physical barrier to prevent migration of bacteria inhibits cell adhesion and facilitates cell migration |
|
|
Aggrecan
|
free proteoglycan
|
|
|
Syndecan and Fibrocan
|
membrane bound proteoglycans
|
|
|
glycoproteins
|
core protein with branched sugars
adhesion molecules that bind to other molecules like heparin sulfate and collagen IV NECESSARY FOR CELL MOTILITY fibronectin, lamin, tenascin, entatin, nidogen |
|
|
intrstitial fluid
|
water, tissue fluid
metabolic support of CT Transport between BVs and parenchyma bound by proteoglycans and hyaluonic acid |
|
|
Edema
|
abnormal build up of interstitial fluid in ECM
Causes: BLocked lymphated Blocked venous return Increased vascular permeability hypertension liver disease starvation |
|
|
Basement Membrane
|
Light microscopy term
Located in ECM on basal aspect of epithelium cells Surrounds all cells (muscle, epithelium, nerve) EXCEPT connective tissue cells |
|
|
EM description of basment membrane
|
Lamina Lucida
Lamina Densa - Collagen Type IV Lamina Reticularis - fine reticular fibers (Type III Collagen) that bind to Lamina Densa |
|
|
Basil Lamina
|
EM term to describe the portion of the basement membrane containing the Lamina Lucida and Lamina Densa
|
|
|
Function of Basement Membrane/Basal Lamina
|
Physical attachment of cells to ECM via ntegrin at the cell surface, the glycoproteins fibronectin, laminin and perlecan, and intactin in the lamina lucida and Collagen IV in the lamina densa and Collagen III in the lamina reticularlis
Acts as macromolecular filter for interstitial fluid being forces ou of and back into capillaries |
|
|
Why is cell motility important
|
Infection
Embryology Wound Repair Metastisis |
|
|
Junctional vs Nonjunctional adhesion
|
Junctional adhesions have specifin morphologically defined regions of the cell membrane
Nonjunctional Adhesions |
|
|
How does a cell know where to leave a blood vessel to reach a site of infection
|
Extravasation - process of attaching to endothelial lining followed by migration of the cell through the capillary wall
|
|
|
How do cells move through the ECM
|
Diapedesis - process of migrating through ECM
|
|
|
What are the 4 steps of Extravasation
|
1. Activation of endothelial cells causes P-Selectine to be displayed on surface
2. Trapping: P-selectin temporarily binds to selectin receptors on leukocytes via H-bonding, causing cell to roll and slow down and bind PAF Adhesion: Activated integrin on leukocyte binds to ICAM of endothelial cell covalently 4. Migration:cell sticks lamellapodia or pseudopodia through to basement membrane and then pulls itself through. Leuk. secretes metalloproteases which breaks apart cellular junctions to slip through |
|
|
How is integrin activated and inactivated
|
Integrin on the leukocyte membrane undergoes a conformational change when the PAF receptor on the leukocyte binds to PAF (platelet activating factor) on the endolthelium. This allows the integrin to bind to ICAM (intracellular adhesion molecule) on the epithelial cell
|
|
|
What are the 2 types of Diapedesis
|
Slow moving: occurs inn fibroblasts and growth cones of neurons (during development)
Mast moving: occus in leukocytes and macrophages, can see cytoplasmic streaming |
|
|
What are the 4 steps of diapedesis
|
1: Extension of lamellipodia (podosomes)
2. Adhesion: formation of focal contacts, attaches cell to substrate which gives cell a point to pull against and holds cell in places 3. Cytoplasmic flow forward, cytoskelitin shifts, cytoplasm goes from gel to sol 4. retraction with footprint, trailing edge thins, forms retraction fiber, "snaps" to leave footprint |
|
|
How does cell membrane move forward during diapedesis (3 possib le mechanisms)
|
1. cytoskeleton elements, actin filaments, are bound to membrane by myosin I. Microfilaments grow, polymerize under influence of profilin to push membrane ahead
2. Myosin I w. plasma membrane attached to it. Microfillaments extended w.o. profilin, but still push membrane forward 3. MyosinI attaches plasma membrane to cytoskeleton and <yosinI moves forward within the membrane to shift microfilaments forward |
|
|
How does cell know which direction to go during diapedesis
|
chemotaxtic factors (released by mast cells)
Calcium concentration gradient |
|
|
S Phase
|
Replication of nuclear DNA
Cells contain the diploid (2n) amount of DNA before S phase and 4n after Duplicate chromosomes consist of 2 identical sister chromatids |
|
|
G2
|
phaseMetabolism of RNA, regulatory proteins and enzymes necessary for mitosis to take place
DNA is analyzed for possible errors and correcte4d before mitosis Cell has two complete diplod sets of chromosomes |
|
|
Mitosis
|
Cell divides
|
|
|
G1 phase
|
Cell volume is restored to normal (2n)
Metabolism of RNA, regulatory proteins, and enzymes necessary for DNA replication Cells can withdrq from the cell cycle into G0 and stop dividing for long periods of time or indefinetley |
|
|
Experimental Systems for Cell Cyle research
|
biochemical analysis of animal eggs and embryos
identification of yeast cell division cycle mutants mammalian cell fusion experiments |
|
|
Mitosis Promoting Factor (MPF)
|
identified from analysis of xenopous eggs
required for progression from G2 into miosis reguired for chromatin condensation, nuclear envelope breakdown, fragmentation of ER and GOlgi, reorginization of microtubules to form mitotic spindleprotein synthesis required for activity A complex of cyclin b and M-Cdk (cyclin dependant kinase) |
|
|
CYclin B
|
Identified in sea urchin embryos
Cyclin B cyclically increases and decreases during post fertilization period Rise and fall just before cleavage parallels MPF activity Protein synthesis required for activity |
|
|
Cdc 25
|
Promotes progress through cell cycle, activates MPF
Deficit leads to elongated cells that don't divide |
|
|
Wee 1
|
Inhibits MPF, inhibits progression through cell cyle
Defecit leads to very small cells that divide to quickyly |
|
|
S-CDKs
|
S phase specific cell cyle promoting factor, diffusible
Identified by mammaliam fusion experiments Critical for preventing re-replication |
|
|
Cyclin dependent kinases (Cdks)
|
activity rises and falls through the cell cycle and lead to cyclic changes in the phosphorylations of intracellular proteins that initiate or regulate the mahor events of the cell cycle
|
|
|
Cyclins
|
bind to Cdks to cyclically regulate their activity. Cdks are dependent on cyclins for their activity . Cyclin levels undergo a cycle of synthesis and degrdation with each cell cycle
|
|
|
How are cyclins regulated through the cell cycle
|
Proteolysis - Ubiquitin dependent
Transcription |
|
|
List and describe the stages of the cell cycle
|
1. Interphase
a. G1: Cell vol. is restored to normal (2n), cell prepares for DNA replication, cells can enter G0 from here and stop dividing b. S Phase: Replication of nuclear DNA (4n) c. G2 phase: cell gets ready for mitosis, dna is analyzed for possible errors and corrected, cell is 4n 2. Mitosis |
|
|
List and describe the stages of mitosis
|
Prophase: chromosomes condense and spindle apparatus forms
Prometaphase: nuclear envelope disappears Metaphase: chromosomes line up on the metaphase plate Anaphase: The chromosomes split and begin to move to the poles Telophase: Cytokenesis occurs, the chromosomes uncoil and the nuclear envelope reforms |
|
|
How and why are Xenopus eggs used to study the cell cycle
|
Why: The eggs are the size of BBs and the embryos are relatively large. After being laid by the female they are "dormant" waiting to be fertilized. After fertilization they undergo rapid synchronous cell division. This dormant period and fertilization period can be tightly controlled and manipulated in the lab.
How: Substances can be injected into the egg to determine their effect on the cell cycle. Or, cell extracts can be prepared from the egg to create a cell-free experiment in the test-tube |
|
|
What are two types of yeast and how are they used to study the cell cycle
|
Saccharomyces cervevisiae - budding yeast
Schizosaccaromyces pombe - fission yeast Conditional mutants in the cell cycle are identified, i.e. temperature sensitive mutants - gene product is active aat he permissive temperature and inactive at the restrictive temperature. Cells can be grown at the permissive temperature and then switched to the restrictive temperature to look at the effects of loss of that particular gene product. Cell division cycle (cdc) mutants will be halted at a specific stage of the cell cycle at the restrictive temperature |
|
|
How are mammaliam cell fusion experiments used to study the cell cycle
|
Cells at different stages of the cell cycle can be fused to look at the resulting effects on the cell cycle. For exampleWhen an S-phase cell and a G1-phase or G2 are fused, the s-phase nucleas will drive the G1 nucleus into s-phase, but not the G2. This indicates that there is a block to re-replication
|
|
|
Mitosis Promoting Factor (MPF)
|
A factor that initiates mitosis
Required for: chromatin condensation, nuclear envelope breakdown, fragmentation of the ER and Golgi, Reorganization fo microtubules to form the mitotic spindle A complex consisting of cyclin B and M-Cdk (cyclin dependant kinase) Levels rise and then fall just before division Regulated by Wee1 Kinase (-) and CD25 Phophatas (+) |
|
|
How do Cyclin dependent kinases (cdks) function in the cell cycle
|
activity rises and fall through the cell cycle
leads to cyclic changes in the phosphorylation of intracellular proteins that intitiate or regulate the major events of the cell cycle. Depemdent on cyclins for their activity. Associate successively with different cyclins to trigger different events in the cell cycle |
|
|
How do cyclins function in the cell cycle
|
bind to Cdks to cyclically regulate their activity.
Required for Cdk activity Undergo a cycle of synthesis and degredation with each cell cycle. |
|
|
What are the 4 classes of cyclins
|
G1/S cyclins - bind Cdks at the end of G1 and commit the cell to DNA replication
S cyclins - bind cdks during s phase and are required for the initiation of DNA replication M cyclins - promote the events of mitosis G1 cyclins - help promote passage through start or the restriction point in late G1 |
|
|
How is Cdk activity suppressed
|
1. phosphorylation in the active site of Cdk inhibits cyclin-Cdk activity; Wee1 phosphorylates, Cdc25 dephosphorylates
2. Cdk inhibitor proteins (CdkIs) bind to the cyclin-Cdk complex and change the conformation of the active site rendering it inactive |
|
|
How are cyclin levels controlled
|
1. Proteolysis via a ubiquitin dependent system
a. SCF ubiquinates G1/S cyclins and some CKIs to control S phase initiation b. APC (anaphase-promoting complex) ubiquinates M Cyclins and other regulators of mitosis 2. transcription |

