![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
173 Cards in this Set
- Front
- Back
|
A
|
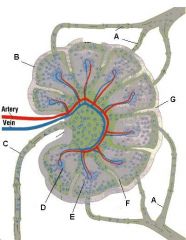
Which letter points to the afferent lymphatic?
|
|
|
C
|
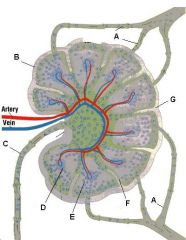
Which letter points to the efferent lymphatic?
|
|
|
The capsule is a fibrous structure which surrounds the lymph node and extends inward to form trabeculae.
|
What is the name of structure B and what happens there?
|
|
|
Germinal center in a follicle of the cortex.
Follicle: Site of B-cell localization and proliferation. Primary follicles are dense and dormant. Secondary follicles have pale central germinal centers and are active. Germinal center: B cells migrate here after being activated. They then change into plasma cells and migrate to the medullary cords. |
What is the name of structure D and what happens there?
|
|
|
Medullary cord, which contains closely packed T-cells and plasma cells (which came from the germinal centers). The cords are separated from each other by medullary sinuses.
|
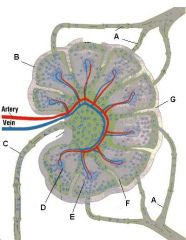
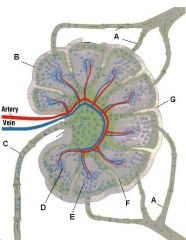
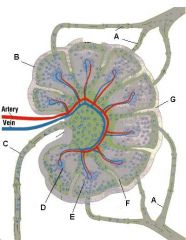
What is the name of structure E and what happens there?
|
|
|
Medullary sinus. These separate the medullary cords and communicate with efferent lymphatics. They contain reticular cells and macrophages.
|
What is the name of structure F and what happens there?
|
|
|
Trabecula. Extension of the fibrous capsule
|

What is the name of structure G and what happens there?
|
|
|
Connective tissue (C is capsule)
|
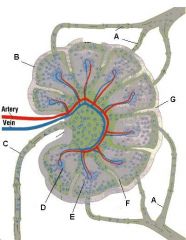
Lymph node: What is A?
|
|
|
Afferent lymphatic vessel with valve
|
Lymph node: What is B?
|
|
|
Capsule
|
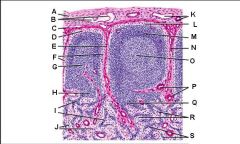
Lymph node: What is C?
|
|
|
Subcapsular (marginal) sinus
|
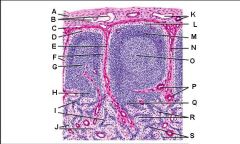
Lymph node: What is D?
|
|
|
Trabecula
|
Lymph node: What is E?
|
|
|
Trabecular (cortical) sinuses
|
Lymph node: What is F?
|
|
|
Germinal center of lymphatic nodule/follicle
|
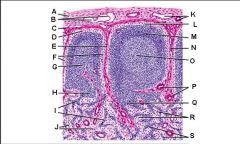
Lymph node: What is G?
|
|
|
Paracortex (deep cortex): Houses T cells. Contains high endothelial venules through which T and B cells enter from blood.
Greatly enlarged in extreme cellular immune response (ie viral). Not well developed in DiGeorge syndrome |
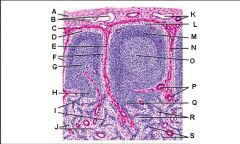
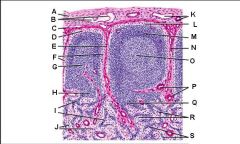
Lymph node: What is H?
|
|
|
Medullary cords
|
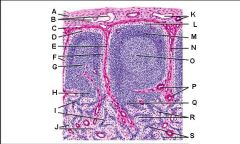
Lymph node: What is I?
|
|
|
Medullary sinuses
|
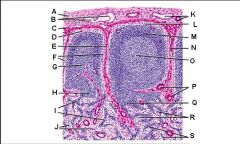
Lymph node: What is J?
|
|
|
Venule and arteriole
|
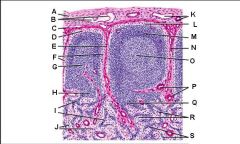
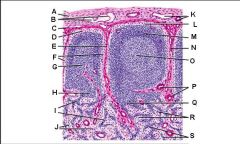
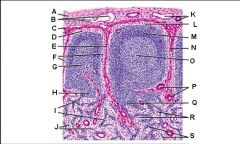
Lymph node: What is K?
|
|
|
Subcapsular marginal sinus
|
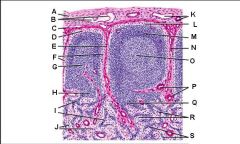
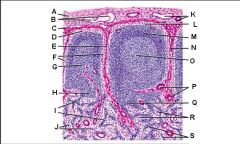
Lymph node: What is L?
|
|
|
Lymphatic nodule/follicle
|
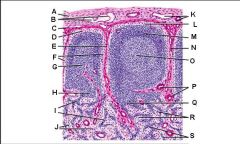
Lymph node: What is M?
|
|
|
Trabecula
|
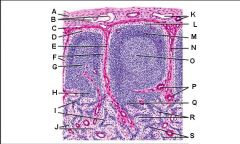
Lymph node: What is N?
|
|
|
Germinal center of lymphatic nodule/follicle
|
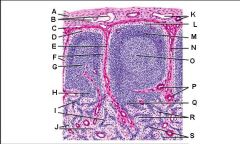
Lymph node: What is O?
|
|
|
Trabecular blood vessels
|
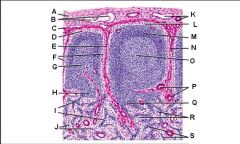
Lymph node: What is P?
|
|
|
Paracortex (deep cortex): Houses T cells. Contains high endothelial venules through which T and B cells enter from blood.
Greatly enlarged in extreme cellular immune response (ie viral). Not well developed in DiGeorge syndrome |
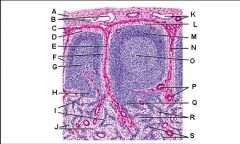
Lymph node: What is Q?
|
|
|
Medullary sinuses
|
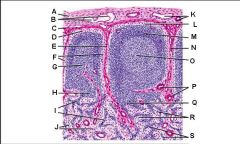
Lymph node: What is R?
|
|
|
Medullary cords
|
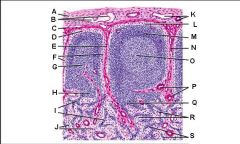
Lymph node: What is S?
|
|
|
Lymph drainage: What does the right lymphatic duct drain?
|
Right arm and right half of head
|
|
|
Lymph drainage: What does the thoracic duct drain?
|
Everything except for the right arm and the right half of head
|
|
|
Splenic sinusoids: What are they?
|
Long, vascular channels in red pulp with fenestrated "barrel hoop" basement membrane and macrophages nearby. Adjacent to splenic cords and contain blood.
|
|
|
How can the spleen be distinguished from a lymph node on histologic section?
|
Spleens have no subscapsular sinus and no cortex or medulla. They have white pulp and red pulp.
|
|
|
What does the white pulp of the spleen contain?
|
Contains:
1. Lymphoid follicles with germinal centers (mostly B cells). Can see aggregation of dark basophilic lymphocytic nuclei. 2. Characteristic central arterioles. Surrounded by a Periarterial lymphatic sheath (PALS) which is a collection of T-lymphocytes. |
|
|
Where is the red pulp of the spleen located?
|
Around and between the lymphatic nodules of the white pulp
|
|
|
Splenic cords: What are they?
|
Structures containing macrophages, plasma cells, lymphocytes, and few RBCs. Separated from each other by splenic sinusoids.
|
|
|
Thymus: Function
|
Site of T-cell differentiation and maturation (T cells differentiate in the Thymus. B cells differentiate in the Bone marrow)
|
|
|
Thymus: Embryological origin
|
Epithelium of 3rd branchial pouches
|
|
|
Lymphocytes: Embryological origin
|
Mesenchyme
|
|
|
Thymus: What does the cortex contain and what does it look like?
|
The lobules resemble lymphatic nodules except they are angular, not round.
Contains: 1. Densely packed (dark) immature T cells. 2. Large epithelial reticular cells which appear as holes within the cortical cells. |
|
|
Thymus: What does the medulla contain and what does it look like?
|
Pale
Contains: Thymic (Hassall's) corpuscles which have a lamellated or whorled appearance due to degenerating epithelial reticular cells. |
|
|
What percentage of T cells which enter thymus survive?
|
2%
|
|
|
What is positive selection of T cells?
|
Retention of T cells that have functioning T cell receptors
|
|
|
Where do positive and negative selection of T-cells occur in the thymus?
|
At the corticomedullary junction
|
|
|
What is negative selection of T cells?
|
Destruction of T-cells that react to self-antigen
|
|
|
Innate immunity vs adaptive immunity: How are receptors that recognize pathogens encoded?
|
Innate: Germline encoded
Adaptive: Undergo VDJ recombination during development |
|
|
Innate immunity vs adaptive immunity: How fast is response to pathogens?
|
Innate: Always fast, no memory response.
Adaptive: Slow on 1st exposure but memory response is faster and more robust. |
|
|
Innate immunity or adaptive immunity: Neutrophils
|
Innate immunity
|
|
|
Innate immunity or adaptive immunity: Macrophages
|
Innate immunity
|
|
|
Innate immunity or adaptive immunity: Dendritic cells
|
Innate immunity
|
|
|
Innate immunity or adaptive immunity: Complement
|
Innate immunity
|
|
|
Innate immunity or adaptive immunity: T cells
|
Adaptive immunity
|
|
|
Innate immunity or adaptive immunity: B cells
|
Adaptive immunity
|
|
|
Innate immunity or adaptive immunity: Circulating antibody
|
Adaptive immunity
|
|
|
T-cell differentiation: Where do T-cell precursors come from and where do they go?
|
From the bone marrow to the thymus
|
|
|
T-cell differentiation: What CD?
|
CD3
|
|
|
T-cell differentiation: What happens to T-cell precursors when they enter the thymus, and what are they called?
|
Once T-cell precursors acquire and display CD4 and CD8, they are cortical thymocytes.
|
|
|
T-cell differentiation: Where is the T-cell in its development when it undergoes positive selection?
|
Both CD4 positive and CD8 positive
|
|
|
T-cell differentiation: Where in the thymus are cells which are positive for both CD4 and CD8 located?
|
Thymic cortex
|
|
|
T-cell differentiation: Where is the T-cell in its development when it undergoes negative selection?
|
Either CD4 positive or CD8 positive, not both
|
|
|
T-cell differentiation: Where in the thymus are cells which are positive for either CD4 or CD8 located?
|
Thymic medulla
|
|
|
T-cell differentiation: What are the two types of helper T cells and where do they differentiate?
|
In the lymph node, helper T cells differentiate into Th1 cells, and Th2 cells.
|
|
|
Differences between Th1 and Th2 cells: Stimulant for differentiation from archetypical helper T cell.
|
Th1: IL-12 from both other Th1 cells and antigen-presenting dendritic cells
Th2: IL-4 from other Th2 cells and presumably an unknown factor from dendritic cells |
|
|
Differences between Th1 and Th2 cells: Cytokines produced by both types
|
Both: IL-2
Th1: IFN-gamma, TNF-alpha Th2: IL-4, IL-5, IL-6, IL-10, IL-13 |
|
|
Cytokine effects: Interleukin 2
|
Stimulates T-cell growth and proliferation
Mnemonic for first 5 interleukins: Hot T-bone stEAk |
|
|
Cytokine effects: Interferon gamma
|
1. Inhibits Th2 cytokines
2. Induces class I and II MHC 3. Stimulates differentiation of monocytes into macrophages. 4. Activates macrophages. |
|
|
Cytokine effects: Tumor Necrosis Factor alpha
|
1. Activates macrophages, neutrophils (also attracts them), and CD8 cells.
2. Induces neutrophil-endothelial cell adhesion. 3. Constitutional: sepsis, cachexia ("wasting away"), fever, acute phase proteins. 4. Tumor cell lysis 5. Increased proliferation of B-cells 6. Increased synthesis of IL-2 receptors by Th cells. 7. Stimulates dendritic cell migration to lymph nodes. |
|
|
Cytokine effects: Interleukin 4
|
1. Growth of B-cells
2. Growth and proliferation of T-cells 3. Synthesis of IgE 4. Class switching of IgG to IgE 5. Inhibits IL-8, IL-1, and TNF-alpha Mnemonic for first 5 interleukins: Hot T-bone stEAk. E as in stimulates IgE production. |
|
|
Cytokine effects: Interleukin 5
|
1. Differentiation of B cells 2. Class switching of IgA
3. Production and activation of eosinophils Mnemonic for first 5 interleukins: Hot T-bone stEAk. A as in stimulates IgA production. |
|
|
What cytokines stimulate the acute phase response?
|
1. IL-1
2. IL-6 3. TNF-alpha |
|
|
What are acute phase response proteins used for?
|
1. Augment immune response (complement, Ig)
2. Regulate the extent of response (protease inhibitors like alpha-1-antitrypsin) 3. Stimulate additional responses (alpha-2-macroglobulin) |
|
|
Cytokine effects: Interleukin 10
|
Big picture: Stimulates Th2 while inhibiting Th1
Specifically inhibits: 1. IL-8 2. IL-1 3. TNF-alpha 4. IFN-gamma |
|
|
What releases: Interleukin 10
|
1. Th2 cells
2. Macrophages |
|
|
Differences between Th1 and Th2 cells: Major effects
|
Both: Downregulate each other
Th1: Activates all lymphocytes and APCs, especially CD8 cells and macrophages. Th2 cells: 1. B cells: Increased differentiation, proliferation, antibody, and class switching. 2: Activation of eosinophils |
|
|
What releases: Interleukin 2
|
Th cells
|
|
|
What releases: Tumor Necrosis Factor alpha
|
Macrophages (emphasized) and Th1 cells
|
|
|
What releases: Interferon gamma
|
Th1 cells (emphasized) and NK cells
|
|
|
What releases: Interleukin 5
|
T cells (especially Th2) and mast cells
|
|
|
What releases: Interleukin 6
|
T cells (especially Th), macrophages, and endothelial cells
|
|
|
What is MHC and what codes for it?
|
Major Histocompatability Complex encoded by Human Leukocyte Antigen (HLA)
|
|
|
What genes code for MHC I?
|
1. HLA-A
2. HLA-B 3. HLA-C |
|
|
What genes code for MHC II?
|
1. HLA-DP
2. HLA-DQ 3. HLA-DR |
|
|
MHC I and II: What cells are they expressed on?
|
I: All nucleated cells except sperm.
II: Antigen Presenting Cells |
|
|
MHC I and II: Where in the cell is antigen loaded onto the MHC?
|
I: RER (mostly intracellular peptides)
II: Acidified endosome |
|
|
B cells and T cells: Effect on Ig
|
B cells: Make it
T cells: (CD4) Help B cells make it and release IFN-gamma to activate macrophages |
|
|
B cells and T cells: Method of killing
|
B cells: IgG opsonizes bacteria and viruses
T cells: (CD8) Directly kills virus-infected cells |
|
|
B cells and T cells: Allergy mechanism
|
B: Type I hypersensitivity, through IgE
T: Type IV hypersensitivity |
|
|
B cells and T cells: Organ rejection speed
|
B: Fast, through antibodies
T: Slow |
|
|
CD/MHC: What binds MHC II?
|
CD4 T-cell receptors
|
|
|
What does MHC I pair with (allosteric interaction)
|
beta2-microglobulin
|
|
|
CD/MHC: What binds MHC I?
|
CD8 T-cell receptors
|
|
|
CD/MHC: What binds CD4 T-cell receptors?
|
MHC II on antigen presenting cells
|
|
|
CD/MHC: What binds CD8 T-cell receptors?
|
MHC I on virus-infected cells
|
|
|
Cytokine effects: Interleukin 1
|
Big picture: Stimulates growth differentiation or product synthesis by T cells, B cells, neutrophils, fibroblasts, and epithelial cells
1. Endogenous pyrogen 2. Activates T cells 3. Upregulates adhesion molecules 4. Induces acute phase reactants 5. Synergizes with TNF-alpha Mnemonic for first 5 interleukins: Hot T-bone stEAk. Hot as in fever. |
|
|
What releases: Interleukin 1
|
1. Professional antigen-presenting cells (macrophages, monocytes, dendritic cells, and B cells)
2. Some non-professional antigen presenting cells (fibroblasts, endothelial cells, others) |
|
|
How does Interleukin 1 cause fever?
|
Steps
1. Migrates to the circumventricular organs 2. Binds with endothelial receptors. 3. Receptors activate Phospholipase A2-COX2-PGE2 pathway 4. Prostaglandin E2's presence in the hypothalamus elevates the thermoregulatory set point and activates neuroendocrine determinants of fever. |
|
|
What are the professional antigen presenting cells?
|
1. Macrophages
2. B cells 3. Dendritic cells |
|
|
What is the CD3 complex?
|
Cluster of polypeptides associated with a T-cell receptor. It is important in signal transduction.
|
|
|
How are Th cells activated?
|
1. APC phagocytoses foreign body.
2. APC presents antigen on MHC II. 3. Signal 1: Th cell's TCR recognizes antigen. 4. Signal 2 (costimulatory): APC's B7 molecule stimulates Th cell's CD28 molecule. 5. Autocrine IL-2 stimulates Th cell to produces cytokines |
|
|
How are Tc cells activated?
|
1. Virus-infected cell presents endogenously synthesized proteins on MHC I.
2. Signal 1: Tc cell's TCR recognizes antigen. 3. Signal 2: IL-2 released from Th cell activates T c cell to kill virus infected cell. |
|
|
Antibody structure/function: What are the components of the heavy chain?
|
Variable: VH
Constant: CH1, CH2, CH3 |
|
|
Antibody structure/function: What are the components of the light chain?
|
Variable: VL
Constant: CL |
|
|
What part of an antibody recognizes antigen?
|
Variable portion of Fab fragment
|
|
|
What part of an antibody fixes complement?
|
Constant part of H chain of IgM and IgG
|
|
|
Antibody structure/function, True or False: Light chain contributes to Fab
|
True
|
|
|
Antibody structure/function, True or False: Heavy chain contributes to Fab
|
True
|
|
|
Antibody structure/function, True or False: Light chain contributes to Fc
|
False
|
|
|
Antibody structure/function, True or False: Heavy chain contributes to Fc
|
True
|
|
|
What is the middle of the variable component of an antibody component chain called?
|
Hypervariable region
|
|
|
Antibody structure/function: Where on an antibody is the hypervariable region?
|
The majority of the variable segments excluding the edges
|
|
|
How are the four chains of an antibody connected?
|
Interchain disulfide bonds at:
1. between the two heavy chains on the Fc side of the hinge region 2. between corresponding light and heavy chains on the Fab side of the hinge region |
|
|
What disulfide bonds does an antibody have?
|
Interchain: Bonds between both heavy chains and between corresponding light and heavy chains
Intrachain: On each segment |
|
|
Where on an antibody is the Amino terminal?
|
At the variable edges of the chains
|
|
|
Where on an antibody is the carboxyl terminal?
|
At the constant edges of the heavy chains
|
|
|
Antibody structure/function: What are the five Cs of Fc?
|
1. Constant
2. Carboxy terminal 3. Complement binding (IgG and IgM only) 4. Carbohydrate side chains 5. Complement binding fragment |
|
|
3 main functions of the antibody
|
1. Opsonization
2. Neutralization (prevents bacterial adherence) 3. Complement activation |
|
|
How is antibody diversity generated?
|
1. Random "recombination" of VJ (light chain) or VDJ (heavy chain) genes.
2. Random combination of heavy chains with light chains 3. Somatic hypermutation 4. Addition of nucleotides to DNA during "genetic recombination" by terminal deoxynucleotidyl transferase. |
|
|
What does terminal deoxynucleotidyl transferase do?
|
Addition of nucleotides to DNA during "genetic recombination" in B cells
|
|
|
What does Tdt stand for?
|
Terminal deoxynucleotidyl transferase
|
|
|
Which immunoglobulins are expressed on the surface of mature B cells?
|
IgM and IgD
|
|
|
What is isotype switching?
|
Differentiation of B cells into plasma cells that secrete IgG, IgA, or IgE
|
|
|
What stimulates isotype switching?
|
Cytokines (IL-4 and IL-5) and CD40-Ligand on T-cells
|
|
|
Main antibody isotype in secondary immune response
|
IgG
|
|
|
Most abundant antibody isotype
|
IgG
|
|
|
Antibody isotypes which cross placenta
|
IgG
|
|
|
Antibody isotypes which fix complement
|
IgG and IgM in the classic complement pathway
Mnemonic: GM makes classic cars |
|
|
Antibody isotypes which opsonize bacteria
|
IgG (and IgA weakly)
|
|
|
Main antibody isotype in primary immune response
|
IgM
|
|
|
Antibody isotypes associated with the J chain
|
IgM and IgA
|
|
|
Antibody isotypes associated with the SP
|
IgA
SP = Secretory Protein |
|
|
Antibody isotype with longest half life
|
IgG (26 days compared with 5 for IgM, the next longest)
|
|
|
Antibody isotype which prevents bacterial/viral attachment to mucous membranes
|
IgA
|
|
|
Antibody isotype found in secretions
|
IgA
|
|
|
Antibody isotype which mediates type I hypersensitivity
|
IgE
|
|
|
How does IgE cause an allergic response?
|
Type I hypersensitivity
1. IgE binds to basophils or mast cells 2. IgE binds antigen 3. These cells release histamine and leukotrienes. |
|
|
Which cells have receptors for IgE?
|
1. mast cells
2. basophils 3. eosinophils 4. monocytes/macrophages 5. platelets |
|
|
Antibody isotype which mediates immunity to worms
|
IgE
|
|
|
Least abundant antibody isotype
|
IgE
|
|
|
Define allotype
|
An individual's allele coding for the constant portions of the antibody's heavy chains.
|
|
|
Define isotype
|
Type of chain in an antibody
Heavy isotypes: alpha, gamma, delta, epsilon, mu Light isotypes: kappa, lambda |
|
|
Define idiotype
|
antibodies of one idiotype share structure of their variable region and thus, antigen binding specificity.
|
|
|
Cytokine effects: Interleukin 3
|
Supports the growth and differentiation of bone marrow stem cells (similar to GM-CSF). Most important during early growth.
Mnemonic for first 5 interleukins: Hot T-bone stEAk. bone, as in bone development |
|
|
What releases: Interleukin 3
|
T cells (emphasis on activated T cells) and thymic epithelial cells
|
|
|
Cytokine effects: Interleukin 6
|
1. Differentiation and growth of B cells and T cells
2. Systemic effects (Acute Phase Response and Fever) 3. Stimulates Ig production |
|
|
What releases: Interleukin 4
|
Th2 cells
|
|
|
Cytokine effects: Interleukin 8
|
1. Major neutrophil chemotactic and adhesion factor
2. Angiogenesis 3. High levels associated with schizophrenia |
|
|
What releases: Interleukin 8
|
1. Monocytes
2. Endothelial cells 3. Fibroblasts |
|
|
Cytokine effects: Interleukin 12
|
1. Promotes differentiation of Th cells into Th1
2. Activates NK cells |
|
|
What releases: Interleukin 12
|
Professional Antigen Presenting Cells:
1. Dendritic cells 2. Macrophages 3. B cells |
|
|
Important cell surface proteins and their functions: Helper T cells
|
1. CD4
2. TCR 3. CD3 (Signal transduction) 4. CD28 and CD40L (Receive costimulatory activation signal respectively from B7 and CD40 which are both on B cells and professional APCs. These two signaling pathways each upregulate the other.) |
|
|
Important cell surface proteins and their functions: Cytotoxic T cells
|
1. CD8
2. TCR 3. CD3 (Signal transduction) |
|
|
Important cell surface proteins and their functions: B cells
|
1. IgM
2. MHC II (Presents foreign antigens to Th cells) 2. B7 and CD40 (Costimulatory activation signal respectively to CD28 and CD40L which are both on T cells. These two signaling pathways each upregulate the other.) 2. CD19, CD21 (Subunits of co-receptor for BCR complex) 4. CD20 (Target in non-Hodgkin's lymphoma of monoclonal antibodies like rituximab) |
|
|
Important cell surface proteins and their functions: Macrophages
|
1. MHC II (Presents foreign antigens to Th cells)
2. CD14 (Works with toll-like receptor 4 to bind lipopolysaccharide. Also a marker for monocytes) 3. Receptors for Fc and C3b (ie opsonins) |
|
|
Important cell surface proteins and their functions: NK cells
|
1. Receptors for MHC I
2. CD16 (subunit of low-affinity Fc receptor [ie. opsonins]) 3. CD56 (adhesion molecule) |
|
|
Important cell surface proteins and their functions: Hematopoietic stem cells
|
CD34 (marker for this type of cell, and a receptor for CD62L, a selectin)
|
|
|
Complement: Viral neutralization
|
C1, C2, C3, C4
|
|
|
Complement: Opsonization
|
C3b
|
|
|
Complement: Anaphylatoxins
|
C3a, C5a
|
|
|
Complement: Neutrophil Chemotaxis
|
C5a
|
|
|
Complement: Membrane attack complex
|
C5b to C9
|
|
|
Deficiency of C1 esterase inhibitor leads to:
|
Hereditary angioedema (overactive complement)
|
|
|
Deficiency of C3 leads to:
|
Severe recurrent pyogenic sinus and respiratory tract infections
|
|
|
Deficiency of C6 through C8 leads to:
|
Neisseria bacteremia
|
|
|
What is Decay Accelerating Factor?
|
Prevents attachment of the alternative complement complex (C3 convertase) to the membrane
|
|
|
When is Decay Accelerating Factor missing?
|
In paroxysmal nocturnal hemoglobinuria
|
|
|
What kind of bacteria does complement defend against?
|
Gram negative bacteria
|
|
|
What activates the classic complement pathway?
|
IgG and IgM.
Mnemonic: GM makes classic cars. |
|
|
What activates the alternative complement pathway?
|
Microbe surface molecules (especially endotoxin)
|
|
|
PGI2
|
Prostacyclin. Vasodilator and inhibits platelet aggregation. Aspirin does not inhibit its synthesis by endothelial cells. Synthesized from PGH2 by prostacyclin synthase in intact endothelial cells.
|
|
|
PGH2
|
Synthesizes PGI2 with prostacyclin synthase in intact endothelial cells. Precursor of thromboxanes. Synthesized from PGG2.
|
|
|
PGE2
|
Vasodilation, pain and fever. Synthesized from PGH2
|
|
|
TxA2
|
Vasoconstriction, platelet aggregation and bronchoconstriction. Coverted from PGH2 by thromboxane synthase.
|
|
|
LTB4
|
chemotaxis and activation of neutrophil adhesion molecules
|
|
|
LTC4
|
Vasoconstriction, increased vessel permeability, bronchoconstriction
|
|
|
LTD4
|
Vasoconstriction, increased vessel permeability, bronchoconstriction
|
|
|
LTE4
|
Vasoconstriction, increased vessel permeability, bronchoconstriction
|

