![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
75 Cards in this Set
- Front
- Back
|
What is a koilocyte?
|
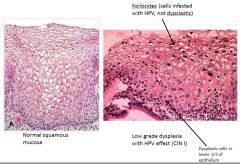
A koilocyte is an HPV infected cell.
The cell gets big and has a lot of cytoplasm; it also has perinuclear halo (space around nuclei). The nuclei are markedly enlarged, dark, and wrinkled (“raisinoid cells”). Frequently you also get binucleation/multiple nuclei. These are infected and not dysplastic cells. Difference between koilocytes and dysplastic cells: Koilocytes tend to be located near the surface. Dysplastic cells are located in bottom 1/3 of epithelium. Koilocytes tend to have a lot of cytoplasm, and dysplastic cells do not. |
|
|
If
Ro = case reproduction ratio B = efficiency of transmission C= rate of change of sexual partners D = duration of infectious Which if the following is the correct equation describing the rate of acquisition or spread of an STI? A) B = RoCD B) C = BRoD C) D = RoBC D) Ro = BCD |
Answer: D) Ro = BCD
|
|
|
Why may those who engage in receptive anal intercourse at a higher risk of transmission of STIs?
|
The mucosal surface of the anus and lower rectum is both more easily traumatized and may contain more target cells (monocytes, macrophages, dendritic cells, lymphocytes).
|
|
|
Can you explain the “epidemiologic synergy” between other STDs and HIV?
|
STDs (both ulcerative and non-ulcerative) may increase the number of target cells (monocytes, macrophages, dendritic cells, lymphocytes) present in genital tract epithelium, thereby increasing the susceptibility of an individual to contract HIV.
|
|
|
The placental cells contain a certain cell type that can act as a target cell for transplacental infection. What is that cell type?
|
Hofbauer cells (macrophages)
|
|
|
Which of the following CANNOT be transmitted perinatally?
A) Chlamydia B) Trichomonas C) HSV D) Parvovirus |
B) Trichomonas
|
|
|
What causes portal hypertension?
|
Portal hypertension results from increased hepatic resistance (extensive fibrosis impaires the passage of blood through the hepatic sinusoids) that could be due to cirrhosis and increased portal venous inflow (splanchnic vasodilation).
|
|
|
What are important non-cirrhotic causes of portal HTN?
|
• Portal vein thrombosis (pre-hepatic resistance to blood flow)
• Sinusoidal Obstruction syndrome (intra-hepatic resistance to blood flow), sometimes called veno-occlusive disease. It’s typically a complication of high dose chemotherapy administered prior to stem cell transplant or from direct radiation to the liver. • Hepatic vein thrombosis – Budd Chiari Syndrome (post-hepatic resistance). Often times, there’s an underlying hypercoagulable disorder. |
|
|
Why does the renin-angiotensin-aldosterone system (RAAS) get activated in portal hypertension?
|
Peripheral and splanchnic vasodilation develops in cirrhotic patients, which leads to lower systemic blood pressure and portal HTN. This activates the RAAS. The resultant increased retention of Na+ leads to fluid retention.
|
|
|
What is hepatic hydrothorax?
|
Hepatic hydrothorax occurs when ascitic fluid travels from the peritoneal space into the chest cavity through tiny holes in the diaphragm that spontaneously develop in some patients. Most of the time (85%) it occurs on the right side.
|
|
|
What is platypnea?
|
Increased shortness of breath when sitting up.
|
|
|
What is the hepatorenal syndrome (HRS)?
|
HRS is renal failure due to renal vasoconstriction caused by deficiency of cardiac output increase in light of a drop in the effective circulating blood volume, which is caused by peripheral and splanchnic vasodilation that occurs in cirrhosis. Patients with HRS always have ascites, hypotension, and frequently hyponatremia (caused by secretion of ADH to increase water retention). HRS is viewed as a terminal event in patients with cirrhosis, and patients have a high risk of dying after this develops.
|
|
|
What’s the difference between type 1 and type 2 hepatorenal syndrome (HRS)?
|
Type 1 happens fast, and type 2 happens slow. (That's basically all we need to know.)
|
|
|
What cocktail of drugs is given for hepatorenal syndrome (HRS) patients?
|
Albumin (increase oncotic pressure)
Octreotide (reduce splanchnic vasodilation) Midodrine (systemic vasoconstrictor) |
|
|
What’s the role of ocretotide in treating varices?
|
Octreotide helps reduce flow through abdomen and helps reduce portal pressure and helps slow down/stop bleeding
|
|
|
Which zone of the liver acinus has a higher concentration of drug metabolizing enzymes?
|
Zone 3 (the centrizonal area) has higher level of phase I drug metabolizing CYP enzymes, which degrade xenobiotics and in the process generate toxic electrophilic metabolites. Zone 3 is more susceptible to injury and is usually the target of toxic effects and drugs.
|
|
|
Which zones of the liver acinus are primarily responsible for cell proliferation following partial hepatectomy?
|
Zones 1 and 2
|
|
|
What are the important functions of zone I/periportal zone in the liver? What about Zone III?
|
• Zone I is responsible for oxidative liver functions (e.g., gluconeogenesis, Beta-oxidation of fatty acids, cholesterol synthesis).
• Zone III is responsible for glycolysis and lipogenesis as well as cytochrome P-450-based drug detoxification. It has the highest concentration of CYP2E1 and is most sensitive to acetaminophen toxicity. |
|
|
In what type of disease would you see hepatocyte Zone II necrosis?
|
Yellow fever
|
|
|
What percent of alcoholics develop liver cirrhosis?
|
• 15-25% develop cirrhosis
• 50% develop some liver damage • 25% are fine (i.e., not affected, genetic resistance) |
|
|
What are some hepatotoxic effects of acetaldehyde?
|
• INCREASES LIPID PEROXIDATION
• BINDS PLASMA MEMBRANES • INTERFERES WITH MITOCHONDRIAL ELECTRON-TRANSPORT CHAIN • INHIBITS NUCLEAR REPAIR • INTERFERES WITH MICROTUBULE FUNCTION • INCREASES COLLAGEN SYNTHESIS (stellate cell activation) |
|
|
What treatment can prevent progression to cirrhosis in a patient with chronic HBV hepatitis?
|
Corticosteroid therapy can prevent progression to cirrhosis in a patient with chronic hepatitis due to HBV.
|
|
|
What are the important non-hepatotropic viruses (i.e., viruses that do not solely infect the liver)?
|
• CMV
• HSV • Adenovirus • EBV None lead to chronic infections. |
|
|
Would you expect to see cirrhosis in a patient with an hepatic adenoma?
|
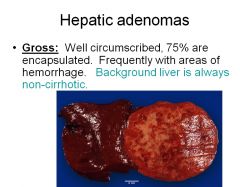
No, the background is always non-cirrhotic. However, fat accumulation is a common feature.
|
|
|
At what cutoff of alpha-fetoprotein would you consider a patient to have HCC?
|
HCC>500 ng/mL
|
|
|
What is Crigler-Najjar Syndrome?
|
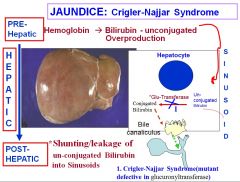
Crigler-Najjar syndrome is a disorder where there’s a mutant defect in the glucuronyltransfersase, which is responsible for transporting unconjugated bilirubin so that it can be conjugated into direct bilirubin. This causes jaundice and the accumulation of unconjugated bilirubin.
|
|
|
What is Gilbert syndrome?
|
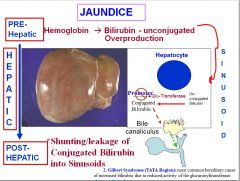
In Gilbert syndrome, the promoter region for the Glu-Transferase is affected, reducing the efficacy of the gene and thus causing jaundice and a buildup of unconjugated bilirubin.
|
|
|
What is primary biliary cirrhosis (PBC)?
|
PBC is a slowly progressing disease characterized by immune-mediated destruction of intrahepatic biliary ductules.
It’s more common in women. The auto-antigen is the E2 subunit of pyruvate dehydrogenase complex, branched chain oxoacid dehydrogenase complex, and 2-oxo-glutaric acid dehydrogenase complex. 90-95% of PBC sera are AMA-positive |
|
|
What oncogene mutations are associated with pancreatic cancer? What tumor suppressor genes are associated with pancreatic cancer?
|
• Oncogenes
o K-ras (most frequently altered oncogene in pancreatic adenocarcinoma, activated by point mutation in >90% of cases) o C-erbB-2 • Tumor Suppressors o p53 o p16 |
|
|
Why are tumors of the head of the pancreas small at time of presentation?
|
Tumors of the head of the pancreas are relatively small at the time of presentation, because they cause compression of the common bile duct leading to jaundice.
|
|
|
How would you define achalasia? What is the pathophysiologic mechanism of achalasia?
|
Achalasia is characterized by:
1) failure of the LES to relax completely with swallowing 2) absent peristalsis in the smooth muscle (thoracic) portion of the esophagus Achalasia results from loss of post-ganglionic inhibitory innervation (nitric oxide mediated) of the smooth muscle esophagus either as a result of parasitic infestation (Chagas’ disease, Trypanasoma cruzi), or, more commonly in the US, as an idiopathic immunologically mediated disease. Clinical manifestations of achalasia may include dysphagia, regurgitation, chest pain, weight loss, and aspiration pneumonia. |
|
|
What are clinical features of primary biliary cirrhosis (PBC)?
|
The retention of components of bile at sites proximal to bile canaliculi and associated lack of bile in the intestine are responsible for most of the symptoms:
* pruritus * fatigability * jaundice * xanthomas (increased cholesterol) * osteoporosis * osteomalacia due to malabsorption of vitamin D * night blindness due to vitamin A deficiency |
|
|
What diseases are associated with primary biliary sclerosis?
|
First of all, you can get the "sicca complex" with PBC - dry eyes and dry mouth (similar to Sjogren's syndrome)
PBC may also b e associated with: Rheumatoid Arthritis Autoimmune thyroiditis Raynaud's phenomenon |
|
|
What cancer is one predisposed to if one has primary sclerosing cholangitis (PSC)?
|
PSC predisposes one to cholangiocarcinoma (160x greater risk).
Also of note, there's a slight male predominance in PSC (2-3:1) as opposed to PBC which occurs more in women. |
|
|
What diseases/disorders are associated with primary sclerosing cholangitis (PSC)?
|
50-80% of PSC is associated with ulcerative colitis and Crohn's involving colon
There are other diseases/disorders, but they're not that important. |
|
|
What are the 3 steps to the formation of gallstones?
|
1) Supersaturated bile: cholesterol is unstable and prone to precipitate
2) Nucleation: cholesterol crystals of cholesterol monohydrate; or dead cellbacteria can start the process 3) Growth: many years to assume gross proportions (like 20-30) |
|
|
Where does the majority of bile salt secretion come from (i.e., synthesis, or whatever)?
|
Enterohepatic circulation. 95% of bile salt secretion is not de novo synthesis but recycling of the bile salts through the enterohepatic circulation.
Bile salts are circulating in the portal circulation. They're secreted through the bile ducts from the liver into the small intestine, reabsorbed in the ileum, and they go back through the portal blood and are taken up by the liver. |
|
|
What are the mechanisms by which alcohol is theorized to cause acute pancreatitis?
|
1. relaxation of sphincter of Oddi, permitting reflux of duodenal contents into the pancreatic duct
2. spasm of the sphincter of Oddi, allowing reflux of bile into the pancreatic duct and sudden release of a large amount of enzymes that are inappropriately activated 3. direct toxic effect of ethanol or its metabolites (e.g., acetaldehyde, which is toxic) 4. obstruction of small ducts by proteinaceous plugs (alcohol changes the composition and concentration of proteins) 5. alcohol sensitizes acinar cells to cholecystokinin |
|
|
What's the name of the enzyme responsible for converting trypsinogen into trypsin?
|
Enterokinase converts trypsinogen into trypsin; trypsin then goes and cleaves other zymogens to activate them.
|
|
|
From what cell type do the majority of pancreatic carcinomas arise?
|
Although the pancreas is mostly acinar tissue, the majority of tumors (90-95%) that arise in the pancreas are of ductal type. Acinar cell tumors are rare (4-5%).
|
|
|
What is the most frequently inactivated tumor suppressor gene in pancreatic cancer?
|
p16 is the most frequently inactivated TSP in pancreatic cancer (inactivated in 95% of cases mainly via deletion and methylation).
p53 mutation is observed in 40-70% of tumors. Important oncogenes= k ras and c-erb-b2 |
|
|
Can you describe how the location of H. pylori infection affects the location of the formation ulcers?
|
Antral gastritis H. pylori infection --> duodenal ulcer
corpus predominant gastritis H. pylori infection --> gastric ulcer |
|
|
What is Hirschsprung's disease?
|
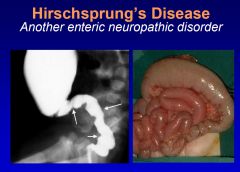
Hirschsprung's disease is a congenital, genetic disorder whose etiopathogenesis involves the failed migration of neural crest cells in the distal hindgut. The absence of enteric neurons (nitric oxide neurons) results in tonic contraction of the distal rectum and colon w/ resultant dilation of the colon proximal to the aganglionc segment. Treatment involves resection of the aganglionc segment of distal bowel.
|
|
|
What is pseudo-achalasia?
|
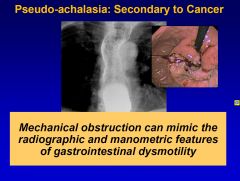
Pseudo-achalasia is achalasia secondary to cancer. That is why you should always check for mechanical obstruction, which can mimic radiographic and manometric features of GI dysmotility, in suspected achalasia patients.
|
|
|
For which inflammatory bowel disease is family history more important?
|
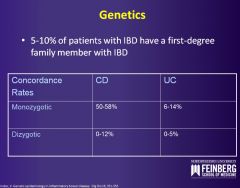
Crohn's disease more commonly has a positive family history.
The associated gene is NOD2/CARD15, a nucleotide-binding oligomerization domain protein 2/caspase recruitment domain protein 15. The protein binds to bacterial cell walls normally and activates NF-kB, stimulating transcription of multiple proinflammatory and protective molecules. Accounts for 20% of Crohn's disease seen in the Caucasian and Jewish population. |
|
|
Can you describe Zollinger-Ellison Syndrome (ZES)?
|
ZES is characterized by peptic ulceration, hypersecretion of gastric acid and an islet cell tumor of the pancreas. The islet cell tumors in the patients have been shown to secrete gastrin. Prolonged high plasma gastrin levels cause an increase of the parietal cell mass with a resultant increased acid secretion. About 60% of the gastrinomas are malignant, 30% benign, and the remaining 10% of pts show only a diffuse hyperplasia of islets.
Symptoms: Related to peptic ulcer (95% of patients). ~40% develop diarrhea |
|
|
What genetic mutation is found in the majority of patients with Peutz-Jegher’s syndrome?
|
Peutz-Jegher’s syndrome is a hereditary, Mendelian dominant, disorder characterized by intestinal polyposis and mucocutaneous melanin pigmentation. 90-100% of patients with a clinical diagnosis of PJS have a mutation in the STK11/LKB1 gene (Serine/threonine kinase 11 (STK11) also known as liver kinase B1 (LKB1) or renal carcinoma antigen NY-REN-19 is a protein kinase that in humans is encoded by the STK11 gene).
|
|
|
Can you describe celiac disease and its pathophysiology?
|
Celiac disease is a malabsorptive disorder due to sensitivity to gluten and/or breakdown products of gluten leading to mucosal damage. Symptoms are related to malabsorption characterized by diarrhea, steatorrhea, weight loss, and development of symptoms related to nutritional deficiency. It is postulated that patients with celiac disease lack a specific mucosal peptidease, resulting in accumulation of gluten or glutamine containing breakdown products that are not hydrolyzed resulting in accumulation of toxic peptides.
|
|
|
What is Ogilvie’s syndrome?
|
Ogilvie’s syndrome is an acquired, acute colonic motility disorder whose pathogenesis is poorly understood but most likely involves disregulation of the autonomic nervous innervation of the hindgut.
|
|
|
What is the pathogenesis of achalasia?
|
In achalasia, there’s an absence of nitric oxide neurons in the myenteric plexus, resulting in tonic contraction of the esophagus.
|
|
|
Where in the gastrointestinal tract are bile salts and vitamin B12 selectively and actively absorbed?
|
The ileum
|
|
|
What would you use to treat Whipple’s disease?
|
Whipple’s disease is due to a bacterium that affects multiple organs, particularly the small intestine, peripheral lymph nodes, and CNS.
Tetracycline can be used to treat Whipple’s disease. |
|
|
What part of the GI tract is predominantly responsible for the absorption of Fe?
|
Duodenum
|
|
|
Which of the following is the most important factor that determines the malignant potential of a colonic adenoma (neoplastic polyp):
A) Grade B) Presence of biomarkers C) Presence of stalk D) Size |
Answer: D) Size
|
|
|
What are symptoms of ulcerative colitis?
|
• Diarrhea
• Passage of blood per rectum • Watery diarrhea • Abdominal cramps |
|
|
What diagnosis is considered in all patients who develop diarrhea while taking antibiotics?
|
Pseudomembranous colitis (PMC)
|
|
|
What is the Rome III Diagnostic criteria for irritable bowel syndrome (IBS)?
|
• Abdominal pain or discomfort at least 3 days per month during the last 3 months associated with 2 or more of the following:
-Improvement (not relief!!) with defecation -Onset associated with a change in frequency -Onset associated with a change in form of stool |
|
|
What levels of gastrin would be considered diagnostic for Zollinger Ellison Syndrome?
|
>200 pg/ mL raises suspicion >1000 pg/mL is virtually indicative of ZES
|
|
|
What are clinical manifestations of secondary peritonitis?
|
• Symptoms
– Abdominal pain, fever, shaking chills, anorexia, nausea, vomiting • Signs – Lies quietly with knees flexed, tachycardia, rapid shallow breathing, marked abdominal tenderness, rebound tenderness, abdominal wall rigidity, hypoactive or silent bowel sounds |
|
|
How does treatment for primary vs secondary peritonitis differ?
|
You treat primary peritonitis with abx primarily and secondary peritonitis is treated with surgery primarily with broad spectrum abx to augment the surgery.
|
|
|
What are the effects of aspirin/other NSAIDs on the risk for colorectal cancer?
|
Aspirin/ NSAIDs have a protective effect as far as risk factors for colorectal cancer goes.
|
|
|
There has recently been a “shift” in the distribution of the location of colorectal cancer. Where are most colorectal cancers found now?
|
There has been a proximal “shift” in the distribution, with a greater proportion of cancers occurring in the right side of the colon, especially the cecum. 50% of cancers occur proximal to the splenic flexure (transverse, ascending and cecum).
|
|
|
How does longstanding ulcerative colitis change your risk for developing colorectal cancer?
|
Longstanding ulcerative colitis increases your risk for developing colorectal cancer. The risk increases after about 10 years of duration of disease and can be as high as 10x the risk of the average population.
|
|
|
A mutation in what gene causes familial adenomatous polyposis?
|
APC in chromosome 5q21-q22.
|
|
|
What are some disadvantages to the use of the fecal occult blood test for colorectal cancer screening?
|
1. Low sensitivity for polyps and cancer (many do not bleed)
2. High rate of false positives (bleeding from other parts of the GI tract, rare meats, etc.) 3. The need to evaluate all positives with colonoscopy |
|
|
If you have Lynch syndrome, what types of cancers can you get, besides colorectal cancer?
|
• Endometrial cancer
• Ovarian cancer • Stomach • Small intestine • Etc. (hepatobiliary tract, upper urinary tract, brain, skin) |
|
|
What are the most common causes of food-borne illness in the U.S., for each major pathogen group (e.g., viral, bacterial, parasitic)?
|
• In the U.S., most common causes of food-borne illnesses:
– Campylobacter spp. (bacterial) – Giardia lamblia (parasitic) – Noroviruses (viral) |
|
|
What is the difference between inflammatory and noninflammatory diarrhea?
|
• Mucous or blood in the stool suggests an inflammatory diarrhea. Also, leukocytes on microscopic exam indicate an inflammatory diarrhea, which results from local tissue destruction by a pathogen that invades the intestinal mucosa. Inflammatory diarrhea more commonly has fever and a longer incubation period.
• Noninflammatory diarrhea is most often toxin-mediated or caused by viruses. No fever, shorter incubation period. |
|
|
Which pathogens produce inflammatory vs. noninflammatory diarrhea?
|
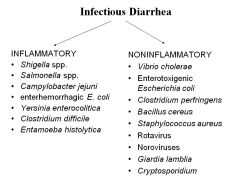
see image
|
|
|
What drugs would you use to treat a Vibrio cholera infection?
|
• Tetracycline and doxycycline (although resistance is increasing)
• Erythromycin and ciprofloxacin are alternatives |
|
|
Which organism causes “gas gangrene” following inoculation into devitalized tissue, and what would you use to treat it?
|
Clostridium perfringens is an anaerobic gram(+) bacillus that causes gas gangrene following inoculation into devitalized tissue. It’s usually self-limiting, so you would not use anything to treat it.
|
|
|
Which bacteria is associated with the ingestion of rice?
|
• Bacillus cereus is a gram(+) rod associated with the ingestion of rice.
• It produces 2 types of toxins. 1 is like LT toxin of E. coli and causes a similar diarrheal illness, except it has ashorter incubation period (6-24 hours). • Toxin 2 resembles the Staphylococcal enterotoxins and likewise causes an illness characterized by vomiting and a short incubation period (1-5 hours). |
|
|
Why would you not prescribe antibtiocs for someone who contracted food poisoning from S. aureus?
|
• Their illness is caused by a preformed enterotoxin, and thus antibtiocs are of no use.
• Common food sources include cream pastries, potato salad, and mayonnaise. |
|
|
Which bacterial infection is rarely followed by Guillain-Barre syndrome, and what would you use to treat it?
|
• Campylobacter jejuni causes diarrhea that is rarely followed by Guillain-Barre syndrome (ascending neuropathy with paresthesias and weakness that may progress to paralysis).
• Fluoroquinolones and macrolides can be used to treat, although resistance to fluoroquinolones is increasing (thought to be due to common use of the antibiotics in animal feed). |
|
|
This bacteria tolerates stomach acid; therefore ingestion of a few hundred bacteria is sufficient for infection. It is transmitted via the five Fs: fingers, food, flies, feces, fomites (inanimate objects such as forks). What is it?
|
• Shigella spp.
• It transcytoses M cells and uses a type III secretion system to invade enterocytes from their basolateral surface. • It also moves through cytoplasm using host actin tails for propulsion (similar to Listeria). |

