![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
97 Cards in this Set
- Front
- Back
|
What is a redox reaction? |
A reaction in which electrons are transferred; oxidation and reduction occurring simultaneously |
|
|
What does an oxidising agent do in a redox reaction? |
Accepts electrons Gets reduced |
|
|
What does a reducing agent do in a redox reaction? |
Donates electrons Gets oxidised |
|
|
What is the oxidation number of uncombined elements? |
0 |
|
|
What is the oxidation number for diatomic molecules? |
0 |
|
|
What is the oxidation number of a monatomic ion, e.g Na+? |
The same as it's charge, e.g. +1 |
|
|
What is the oxidation number of a compound ion, e,g. SO4^2- |
The same as it's charge, e.g. -2 |
|
|
What is the sum of the oxidation numbers in a neutral compound? |
0 |
|
|
What is the oxidation number of combined oxygen? What is the exception to this rule? |
-2 Except in peroxides(e.g. H2O2) - then it is -1 |
|
|
What is the oxidation number of hydrogen? What is the exception? |
+1 Except in metal hydrides e.g. NaH where is has to be -1 |
|
|
How can the oxidation number of an element be made clear through the chemical name? |
Roman numerals E.g. Copper (II) sulphate |
|
|
What causes the oxidation number of an element to decrease? |
Gain of electrons |
|
|
What causes the oxidation number of an element to increase? |
Loss of electrons |
|
|
What is the reaction called if the same element is oxidised and reduced at the same time? |
Disproportionation reaction |
|
|
Why does ionisation energy decrease down the group? |
Each element down the group has an extra electron shell The extra inner shells shield the outer electrons from the attraction of the nucleus They are also further away from the nucleus, which reduces the nucleus's attraction This makes it easier to remove electrons Which reduces the ionisation energy |
|
|
What happens when group 2 elements are reacted with water? |
Gives a metal hydroxide and hydrogen |
|
|
Why do group 2 elements get more reactive down the group? |
Ionisation energies decrease |
|
|
What happens when group 2 elements are burnt in oxygen? |
Produce characteristic flame colours Form solid white oxides |
|
|
What is the flame colour of lithium? |
Red |
|
|
What is the flame colour of sodium? |
Orange/yellow |
|
|
What is the flame colour of potassium? |
Lilac |
|
|
What is the flame colour of rubidium? |
Red |
|
|
What is the flame colour of caesium? |
Blue |
|
|
What is the flame colour of calcium? |
Brick-red |
|
|
What is the flame colour of strontium? |
Crimson |
|
|
What is the flame colour of barium? |
Green |
|
|
How would you conduct a flame test? |
Mix a small amount of the compound you're testing with a few drops of hydrochloric acid Heat a piece of platinum or nichrome wire in a hot Bunsen flame to clean it Dip the wire in the compound/acid mixture Hold it in a very hot flame and note the colour produced |
|
|
Why do group 1 and 2 compounds produce colours when burnt in oxygen? |
The energy absorbed from the flame causes electrons to move to higher energy levels. When the electrons fall back down to lower energy levels, energy is released in the form of light. The difference in energy between the higher and lower energy levels changes the wavelength of the light, which changes the colour observed |
|
|
What happens when group 2 elements react with chlorine? |
Forms solid chlorides |
|
|
Why do the oxides of group 2 elements form bases? |
They react readily with water to form metal hydroxides, which dissolve. The hydroxide ions (OH-) make these solutions very alkaline
Exception- magnesium oxide |
|
|
What is the trend in solubility of metal hydroxides as you go down group 2? |
Become more soluble as you go down the group (This in turn means they form more strongly alkaline solutions) |
|
|
What is the trend in solubility of metal sulfates as you go down the group? |
They become less soluble as you go down the group. Barium sulfate is insoluble |
|
|
How does the thermal stability of the nitrates change as you go down the group? |
As you go down the group, the nitrates become more thermally stable |
|
|
What is the colour of chlorine in an organic solvent (e.g. Hexane) |
Virtually colourless |
|
|
What is the colour of iodine in an organic solvent (e.g. Hexane)? |
Pink/violet |
|
|
Why does thermal stability of the nitrates increase down the group? |
The nitrate ions are large and can be made unstable by the presence of a cation (+). The cation polarises the anion, distorting it. The larger the cation, the greater the distortion, and the greater the distortion, the less stable the anion |
|
|
Why are group 2 compounds less thermally stable than group 1 compounds? |
The greater the charge on the cation, the greater the distortion and the less stable the carbonate/nitrate ion becomes. Group 2 have a greater positive charge |
|
|
What is meant by 'thermally stable'? |
You can't heat them enough with a Bunsen burner to make them decompose (though they will decompose at higher temperatures) |
|
|
What do group 1 nitrates decompose to form? |
The metal's nitrite and oxygen E.g. 2KNO3 --> 2KNO2 + O2 |
|
|
What do group 2 carbonates decompose to form? |
The oxide of the group 2 metal and carbon dioxide E.g. CaCO3(s) --> CaO(s) + CO2(g) |
|
|
What do group 2 nitrates decompose to form? |
The metal's oxide, nitrogen dioxide and oxygen E.g. 2Ca(NO3)2(s) --> 2CaO + 4NO2(g) + O2(g) |
|
|
How can the thermal stability of carbonates be measured? |
Measure how long it takes for carbon dioxide to be produced (using lime water) |
|
|
What colour is fluorine in its standard state? |
Pale yellow |
|
|
What colour is chlorine in its standard state? |
Green |
|
|
What colour is bromine in its standard state? |
Red-brown |
|
|
What colour is iodine in its standard state? |
Grey |
|
|
How does the electronegativity of the halogens change as you go down the group? |
It decreases |
|
|
Why do halogens have low solubility in water? |
They are covalent molecules |
|
|
What is the colour of bromine in water? |
Yellow/orange |
|
|
What is the colour of bromine in hexane? |
Orange/red |
|
|
What is the colour of iodine in water? |
Brown |
|
|
What is the colour of iodine in hexane? |
Pink/violet |
|
|
How does the reactivity of the halogens change as you go down the group? |
It decreases |
|
|
Why does the reactivity of the halogens decrease as you go down the group? |
The atoms become larger, so the outer electrons are further away from the nucleus. The outer electrons are also shielded from the attraction of the positive nucleus because there are more inner electrons. This makes it harder for the larger atoms to form an ion, making them less reactive |
|
|
What happens to the oxidising power of the halogens as you go down the group? |
They become less oxidising |
|
|
What is a disproportionation reaction? |
When the same element is oxidised and reduced in the same reaction |
|
|
What happens when chlorine reacts with COLD sodium hydroxide (NaOH) ? |
Produces NaClO + NaCl+ H2O ^(Sodium chlorate) |
|
|
What happens when iodine reacts with HOT sodium hydroxide? |
Produces NaIO3 + NaI + H2O ^(sodium iodate (III)) |
|
|
What is the general reaction between a halogen (X2) and a cold alkali solution (e.g. KOH)? |
X2 + 2KOH => KXO + KX + H2O |
|
|
What is the general reaction between a halogen (X2) and a hot alkali solution (e.g. KOH)? |
3X2 + 6KOH => KXO3 + 5KX + 3H2O |
|
|
What is the formula for a chlorate (I) ion? |
(ClO)- |
|
|
What is the formula for a bromate (III) ion? |
(BrO2) - |
|
|
What is the formula for an iodate (V) ion? |
(IO3)- ion |
|
|
What is are the trends in thermal stability in group 1 and 2 carbonates?
|
Thermal stability increases down the group- Lithium is the group 1 carbonate to decompose under a bunsen flame, group 2 are all slightly less thermally stable
|
|
|
How would you minimise the sources of measurement uncertainty in titrations?
|
Repeat titrations and take an average
Calibrate equipment Wash the burette with the titre liquid before starting |
|
|
How is percentage uncertainty calculated?
|
[(Experimental value - Accepted value)/Accepted value]x100%
|
|
|
What happens in the reaction of chlorine with iron (II) ions in solution?
|
The chlorine is a powerful oxidising agent, so the iron (II) ions are oxidised to iron (III) ions. The chlorine is reduced to chloride ions in the process
|
|
|
What is observed in the reaction of potassium halides with concentrated sulphuric acid?
|
With KF and KCl, the halide ions aren't strong enough reducing agents to reduce the sulphuric acid, and all that is produced is steamy fumes of a hydrogen halide.
For KBr, the bromide ions are stronger reducing agents and can reduce the H2SO4 to Br2, SO2 and H2O. For KI, the iodide ions are strong reducing agents and can reduce the H2SO4 to I2, H2S and H2O. |
|
|
What is observed in the reaction of potassium halides with halogens?
|
Iodine has no effect.
Bromine only has an effect on KI (turns orangey brown) Chlorine has an effect on KBr (turns yellow) and KI (turns brown-green) |
|
|
What is observed in the reaction of potassium halides with silver nitrate solution?
|
KCl- White ppt.
KBr- Cream ppt. KI- Yellow ppt. |
|
|
What is the reaction with silver halides and sunlight?
|
Decomposes into silver and halide ions
|
|
|
What are the solubilities of silver halides in aqueous ammonia solution?
|
Silver chloride will dissolve in ammonia solution to give a colourless solution
Silver bromide will not dissolve in dilute ammonia solution, but will dissolve in concentrated ammonia solution Silver iodide will remain insoluble in ammonia solution of any concentration |
|
|
What would you expect the properties of fluorine and its compounds to be, based on the trends of the halogens?
|
undefined
|
|
|
What would you expect the properties of astatine and its compounds to be, based on the trends of the halogens?
|
undefined
|
|
|
How does concentration affect the rate of a reaction? Explain why
|
undefined
|
|
|
How does temperature affect the rate of a reaction? Explain why
|
undefined
|
|
|
How does pressure affect the rate of a reaction? Explain why
|
undefined
|
|
|
How does surface area affect the rate of a reaction? Explain why
|
undefined
|
|
|
How do catalysts affect the rate of a reaction? Explain why
|
undefined
|
|
|
What is collision theory?
|
undefined
|
|
|
What is the Maxwell-Boltzmann model?
|
undefined
|
|
|
How can the Maxwell-Boltzmann model be used to relate changes of concentration and temperature to a change in the rate of a reaction?
|
undefined
|
|
|
What is activation energy?
|
undefined
|
|
|
What is the relationship between catalysts and the effect of temperature changes to the rate of reaction?
|
undefined
|
|
|
What is the role of a catalyst in a reaction?
|
undefined
|
|
|
Why are chemical equilibria said to be dynamic?
|
undefined
|
|
|
What is the effect of a change of temperature on the position of equilibrium in a reaction?
|
undefined
|
|
|
What is the effect of a change of pressure on the position of equilibrium in a reaction?
|
undefined
|
|
|
What is the effect of a change of concentration on the position of equilibrium in a reaction?
|
undefined
|
|

What effect would a change in temperature have on the equilibrium position for this exothermic forward reaction?
|
An increase in temperature would shift the equilibrium to the left
A decrease in temperature would shift the equilibrium to the right |
|

What effect would a change in pressure have on the equilibrium position for this reaction?
|
undefined
|
|

What effect would a change in concentration of chlorine have on the position of the equilibrium for this reaction?
|
undefined
|
|
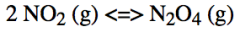
What effect would a change in temperature have on the position of the equilibrium for this (exothermic) reaction?
|
An increase in temperature would result in the equilibrium being shifted towards the left- more of the NO2 is produced
A decrease in temperature would result in the equilibrium being shifted towards the right- more of the N2O4 is produced |
|
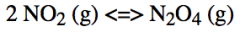
What effect would an increase in pressure have on the position of the equilibrium of this reaction?
|
The equilibrium would be shifted to the right to balance the pressure
|
|
|
What is Le Chatelier's principle?
|
undefined
|
|
|
What is the reaction between hydrogen halides and ammonia?
|
HX + NH3 -> NH4X
|
|
|
What is the reaction between hydrogen halides and water?
|
HX + H2O-> X- + H3O+
|

