![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
383 Cards in this Set
- Front
- Back
|
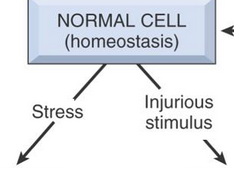
What are some useful things I should do to prepare for boards and rotations?
|
1. Do some UWorld questions every day for a long time
2. Study on a tangent and let your mind cnnect the dots to other systems 3. Read Robbins |
|
|
What kind of group dynamic tends to forms closer to boards time?
|
people commiserate with one another over how hard and ridiculous everything is.
Don't be one of those people! |
|
|
What attitude do people tend to take toward med school and what attitude should we take?
|
people And to think about it as something to endure when really it is something that we should be grateful for
|
|
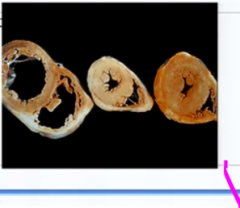
what is happening to the cells in the heart on the left? Why?
|
They are stretching out and gaining more muscle longitudinally because of greater volume stretching out the heart
|
|
|
Are there more cells? How do know?
|
No, because cardiac and skeletal muscle and nerves only undergo atrophy and hypertrophy, not hypoplasia and hyperplasia.
|
|
|
What do cells lose in order to shrink when they become atrophic?
What do they gain in hypertrophy? |
Same thing - Organelles and cytoskeleton
|
|
|
What process usually comes with hyperplasia?
|
Hypertrophy
|
|
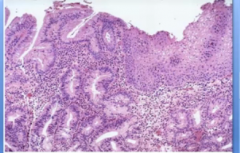
What is this?
|
metaplasia
|
|
|
Why would cells undergo metaplasia?
|
Because they want to become better suited to a new environment
|
|
|
Why would somebody with Barrett's esophagus all of a sudden stop feeling pain?
What would the patient have to worry about then? |
If the metaplasia got so bad that it became dysplasia and then cancer, destroying the nerve roots
the new threat would be choking on your food from it not going down |
|
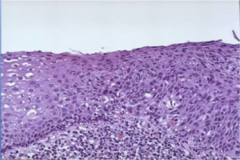
What is this?
|
invasive dysplasia
|
|
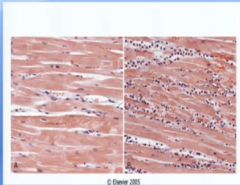
Which is the control? How do you know?
|
The one on the left because you can still see nuclei in the cells
|
|
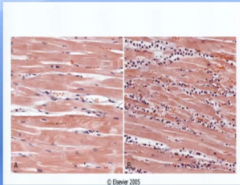
Why are there so many purple dots in the right picture?
|
They are immune cells coming to repair or cleanup the damaged cells
|
|
|
What is the #1 cause of cellular injury?
|
hypoxia
|
|
|
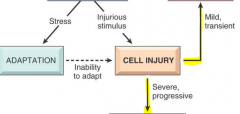
List the four things I need to after in pathology from cause to ultimate effect.
How many things are there to learn in each category? |
Injury
pathogenesis morphology clinical they're just a couple types of injury, but each step up create exponentially more possibilities. That's why it's so important to get a good foundation |
|
VERY IMPORTANT ROADMAP FOR CELL INJURY! TRY TO RECITE IT COLD BEFORE GOING THROUGH THIS.
|
|
|
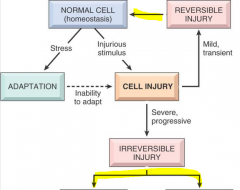
|
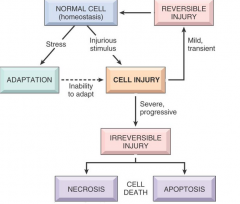
|
|
|
What stage what a heart that is thiCK from hypertension in?
|
adaptation
|
|
|
What are the different types of adaption that cells can go through? 5
|
Atrophy, hypertrophy, metaplasia, hypoplasia, and hyperplasia
|
|
|
What are the two things that happen to produce atrophy?
|
Decrease in cell number and size
|
|
|
Is typically how do cells decrease in number in atrphy?
|
apoptosis
|
|
|
Describe how cytoskeleton and organelles are removed to shrink the cell.
What are these processes called? |
Organelles – autophagy – sells and send their own lysosome to kill and breakdown organelles for parts
cytoskeleton – cytoskeleton is marked with ubiquitin which signals proteosomes To come and break it down |
|
|
How come we say atrophy is a decrease in size and number, but hypertrophy is just an increase in size?
Use muscle as an example |
There is not a limitation to decrease in cell size and number, but certain tissues cannot undergo hyperplasia. However, in tissues without this restriction, hypertrophy and hyperplasia usually come together.
In disuse atrophy, people can never regain back full muscle strength because there was a decrease in cell number. However, we don't gain any more muscle cell from working out a lot |
|
|
Where do we get new cells from in hyperplasia?
What does this explain about the limitation of heart, skeletal, and nervous tissue? |
From stem cells, Which these tissues don't have
|
|
|
Can prosthetic hyperplasia and endometrial hyperplasia become cancerous?
|
Only endometrial. That is why they call it BENIGN prostatic hyperplasia
|
|
|
What structures increase in BPH?
|
Both glands and muscle
|
|
|
What is the risk for all metaplasia is? What is the exception?
|
The progression to dysplasia and then invasiveness
The exception is the apocrine cells of the breasts |
|
|
What metaplasia is deeply expected from women and why? And when?
|
Metaplasia of the Endo cervix during puberty because the cervix is suddenly exposed to the acidic environment of the vagina during puberty.
|
|
|
What does this explain about recommended pap Smear schedules?
|
You should get one at 21 to check for dysplasia. If you don't have it then, then you only need to come back once every three years because that means that your body is handling the metaplasia relatively well after all your teen years
|
|
|
How can you compare dysplasia to metaplasia?
|
Dysplasia is the dark side of metaplasia. It is marked by the disorganized arrangement of the cells
|
|
|
What is the nucleus: cytoplasm ratio of cells progressing from their blast stage to maturity? Why?
|
Decreases because the mature cells have already undergone all their growth stages so the DNA can collapse and make more room for the functional organelles in the cytoplasm
|
|
|
What happened to the nucleus: cytoplasm ratio in dysplasia ? Why?
|
Because the cell are all ready and ways to divide and switch morphologies
|
|
|
What is the next stage that dysplasia can go to? When does it do this?
|
Invasive cancer when it crosses the basement membrane
|
|
|
What are two examples of the leading causes of dysplasia in America?
|
Smoking and HPV, which put a lot of stress on cells
|
|
|
In the beginning of metaplasia, where would you see the new morphology of cells? Why?
|
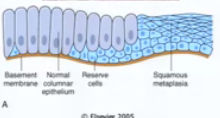
Near the basement membrane or wherever stem cells are because they are the ones that form new cells
|
|
|
Can you describe metaplasia in a metaphor?
How should you describe it to a patient? |
Metaphors are great to use for patient because then they will understand it better.
Metaplasia in the DOUBLE-EDGED SWORD, and in most cases, represent and UNDESIRABLE change, however protective |
|
|
What will question met a plastic cells into malignant transformation? What is the exception?
|
If the stresses that started the metaplasia continued. Exception, as always, is apocrine metaplasia of the breast
|
|
|
Where does the Anterior communicating artery happen? What structures nearby affected by a berry aneurysm?
|
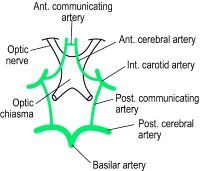
Right in front of the optic chiasm between the cavernous sinus is.
The center optic chiasm and the most superior nerve in the cavernous sinus (CN 3) |
|
|
How does the internal carotid travel once it reaches the skull?
|
In a U-shaped through the cavernous sinus
|
|
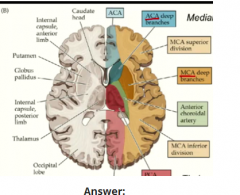
How can you tell by the structures around it that the interior limb carried limbic information to the frontal cortex and cerebellar information to and from the frontal cortex?
|
Limbic – notice that is right to the hypothalamus which is in front of the 3rd ventricle
cerebellar motor planning – carry those denticulate neuron and the interior limb to the forebrain where we plan our action |
|
|
How do you know that the carrier branch supplies thalamus?
|
Since it branches off the Basilar artery at the front of the brainstem, it must travel backward and hit the thalami that it right on top of the midbrain like a pair of eggs
|
|
|
Where is the cingulate gyrus in comparison to the fornix?
|
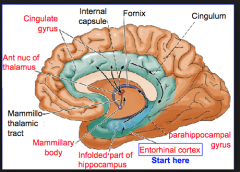
It is above the fornix and the lateral ventricle and the corpus callosum considered part of the "cortex"
|
|

label this
|

cingulate gyrus, fornix, hippocampus
|
|
|
WHat are The 8 etiologies of cellular injury?
|
1. Hypoxia
2. Chemical agents 3. Infectious agents 4. Physical agents 5. Genetics 6. Immune reactions 7. Nutritional Imbalances 8. Aging |
|
|
What are 5 broad causes of hypoxia?
|
1. blood coming in is blocked
2. old blood cannot leave 3. there is not enough blood to perfuse (tube is empty) 4. the blood cannot carry any oxygen 5. there is no oxygen being loaded onto the blood |
|
|
How much time can your cell spend without oxygen before suffering irreersible damage?
|
20 minutes
|
|
|
What are the two main etiologies of disease?
|
hypoxia and free radical damage
|
|
|
what is the hallmark of reversible cell damage?
|
cellular swelling
|
|
|
what causes the swelling
|
low ATP not being able to maintain the Na/K pumps
|
|
|
What ions become high vs low in the cell? (3)
|
high Na and Ca
low K |
|
|
Why does calcium rise? Where does it comes from initially and the later?
|
there are also ATP calcium pumps to remove it, which aren't working now.
Ca comes initially from the extracellular space, then from the mitochondria and ER |
|
|
What does excess calcium do to the cell?
|
it activates enzymes
|
|
|
name these enzymes and what process they will initiate.
|
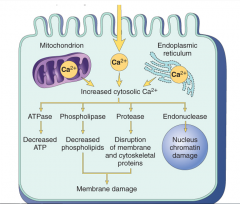
phospholipases also induce inflammation
also increases apoptosis from activating caspases and increasing mitochondria membrane permeability |
|
|
How do we know that calcium causes all this damage?
|
The importance of Ca2+ in cell injury was established by the finding that depleting extracellular Ca2+ delays cell death after hypoxia and exposure to some toxins.
|
|
|
WHy do we inject magnesium into people with IHD and stroke and gangrene? (or any hypoxic process for that matter)
|
it competes with calcium for the calcium channels and wins because it is more electronegative.
|
|
|
Why is it more electronegative?
Why does it go through the same channel as calcium? What other example in the body can you think of that utilizes this principle? |
they are both 2+. but magnesium is higher up on the periodic table and so has 1 less electron shell and is more electronegative.
The in between cell channels of the thick ascending tubule. |
|
|
So what kind of electrolyte balances would someone taking furosemide get? (3)
|
1. hyponatremia
2. hypocalcemia 3. hypomagnesemia |
|
|
WOuld the hypocalcemia or hypomagnesemia be worse? Why?
|
hypocalcemia because magnesium would still be preferentially absorbed
|
|
|
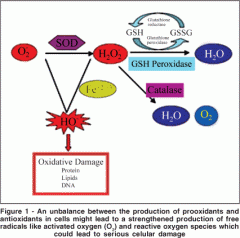
Define ROS's
|
Reactive oxygen species (ROS) are a type of oxygen-derived free radical whose role in cell injury is well established.
|
|
|
What do free radicals do damage to? How does this progress?
|
they oxidize
1. inorganic stuff 2. proteins 3. lipids (membrane) 4. nucleic acids (DNA) it turns these things into free radicals which will turn something else into a free radical and so on |
|
|
How are ROS's usually produced and dealt with in the body?
|
They are produced normally in cells during mitochondrial respiration and energy generation, but they are degraded and removed by cellular defense systems.
|
|
|
How may ROS's start dealing damage? (2)
What is THE STATE OF having too many free radicals called? |
1. When the production of ROS increases
or 2. the scavenging systems are ineffective, the result is an excess of these free radicals, leading to a condition called OXIDATIVE STRESS. |
|
|
What pathophysiologies involve free radical damage? (6 examples)
|
1. ischemia-reperfusion
2. chemical and radiation injury 3. toxicity from oxygen and other gases 4. cellular aging 5. microbial killing by phagocytic cells 6. Tissue injury caused by inflammatory cells. |
|
|
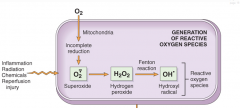
How does a free radical result from normal mitochondria function?
|
ATP generation relies on sending 4 electrons down the energy chain to finally have cytochrome c place it on an O2 molecule acccross the membrane in order to later be reduced to 2 H2O. There are free radical intermediates created by this that may escape without being fully oxidized.
|
|
|
WHat free radicals are created in this normal process of oxidative phosphorylation?
(3 in successive steps) |
|
|
|
Show what oxygen normally looks like with it's bonds
|
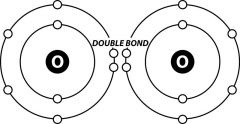
|
|
|
What happens to oxygen to make it superoxide?
|
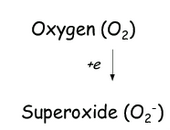
one electron from mitochondria
|
|
|
What does hydrogen peroxide and it's bonds look like?
|
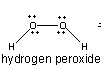
|
|
|
Run through the organic chemistry of how oxygen is reduced to 2H2O in the cells.
|
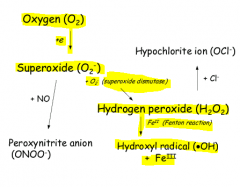
not displayed is that an OH- ion is also produced and that is protonated to water.
|
|
|
WHat kinds of processes can cause free radical creation? (6)
|
1. normal cellular respiration that is unbalanced
2. reduced transition metals 3. inflammation processes 4. endogenous metabolism of toxic substances 5. radiation 6. nitric oxide production |
|
|
What is the energy gained by the transfer of an electron from cytochrome c to oxygen used for?
What makes the ATP? |
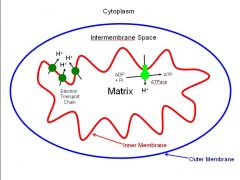
to pump hydrogens from the matrix accross the inner mitochondrial membrane to the intermembrane space, where they will flow back into the matrix to spine the ATPase to make ATP!
|
|
|
What oxidation state of iron do we normally have in the blood?
What is this called? How can you use rust to remember the oxidation state? |

Fe3+ - FerrIC iron
Rust is red like blood and forms from oxidation. Therefor normal iron is n the more oxidized 3+ rather than 2+ state. (ferrous Fe2+ is black) |
|
|
How do you name metals of different oxidation states? Mnemonic?
Give examples in Fe and Cu. |
use the suffixes -ous and -ic. The suffix -ic will indicate the ion of higher ionic charge:
Fe2+ ferrous ion Fe3+ ferric ion Cu+ cuprous ion Cu2+ cupric ion "ICK! oxidation is so bad for you!" |
|
|
Give the system and mnemonic for naming oxyanions.
|
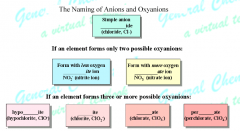
You are a HYPOcrITE!
hypo= low so ite will have less oxygens because they are paired |
|
|
How do you name the acids of oxyanions? mnemonic?
|
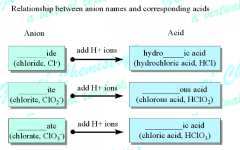
ICK! more oxidation from more oxygens! So -ATE will become -IC to correspond with the naming of metals with higher oxidation states
|
|
|
WHy are transition metals in their reduced state capable of producing free radicals?
|
they can cause the fenton reaction to produce hydroxyl radical
|
|
|
What does the hydroxyl radical look like with it's elctrons in valence?
|
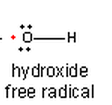
|
|
|
Show the feton reaction.
Mnemonic for necessary reactant? |
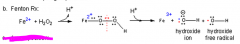
Fe (FEnton) 2+ will pull H2O2 apart and donate an electron to only one of the pieces, leaving itself oxidized to 3+
|
|
|
Why can radaation cause free radical damage?
What kind of radiation does this? 2 examples What is the reaction it causes? |
The absorption of radiant energy (e.g., ultraviolet light, x-rays).
Ionizing radiation can hydrolyze water into hydroxyl (OH•) and hydrogen (H•) free radicals. |
|
|
Is this how it causes skin cancer?
|
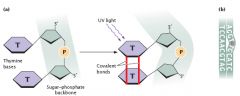
not exactly, that has more to do with making thymine dimers and causing DNA mutation
|
|
|
what exogenous chemicals can cause free radicals when metabolized?
What organ did this deal damage to and why? |
carbon tetrachloride in the liver because that was where it was metabolized
|
|
|
What did carbon tetrachloride used to be in?
|
dry cleaning solutions and refrigerants
|
|
|
WHy would inflammation cause free radicals? Name the process.
Which two free radicals? |
Respiratory burst (sometimes called oxidative burst) releasing (superoxide radical and hydrogen peroxide) from immune cells, especially neutrophils and monocytes
|
|
|
What defenses do some bacteria have to hydrogen peroxide?
What enzyme do we have for this? |
catalase to turn it into O2 and H2O
also catalase! |
|
|
What is the central part of catalase that makes it functional?
Think about the fenton reaction. |
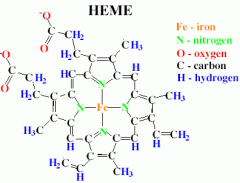
you need a transition metal to pull apart hydrogen peroxide
it has a porphyrin ring |
|
|
Show the net stoichiometry of a catalase reaction
|
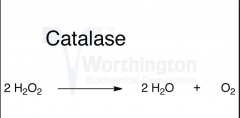
|
|
|
How can NO cause free radicals?
|

It can act as a free radical or can be converted into highly reactive nitrite species
the oxygen is missing one electron for a complete octet |
|
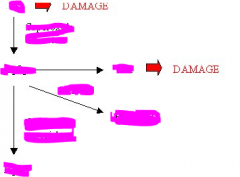
|
|
|
|
Same thing as before. Try to imagine it yourself by reciting.
THIS IS VERY IMPORTANT FOR IMMUNOLOGY, MICROBIO, PATHOLOGY, AND BIOCHEMISTRY! |
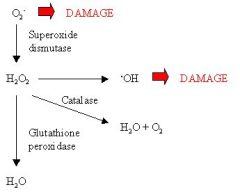
|
|
|
how do you remember that catalase creates H2O AND O2?
|
because when you drip H2O2 on catalase positive bacteria, they produce oxygen bubbles with water over and under it!
|
|
|
What is the way we deal with H2O2 in the mitochrondria? How do you deduce this?
|
with glutathione peroxidase because that produces water and that is the end product of cellular respiration.
|
|
|
What does glutathione add to the H2O2?
How does glutathione replenish what it lost in the process? |
two hydrogens to make it 2H2O
Adding hydrogens will reduce H2O2 Gets hydrogens from NADPH + H+ donated to it |
|
|
Where do we get the H+ and electrons to put onto water?
|
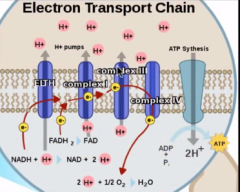
from NADH and FADH2 gathered. from the TCA and Glycolysis fed through the ETC.
electron given by cytochrome C H+ given by ATP synthetase |
|
|
What process do NADH and FADH2 come from?
|
2 NADH from glycolysis and the rest from TCA
|
|
|
What happens to NADH produced in glycolysis in respiration?
in anaerobic respiration? |
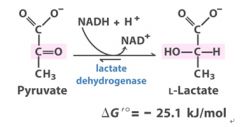
it must be shuttled via malate or DHAP through the inner mitochondrial membrane to be delivered to the TCA.
In anaerobic respiration, NAD+ is regenerated by tossing the electron and hydrogen onto pyruvate to reduce it to lactate |
|
|
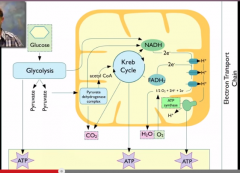
When does carbon dioxide come out during respiration?
|
1 pair when pyruvate --> acetyl COA
2 pairs during the TCA cycle |
|
|
|
|
|
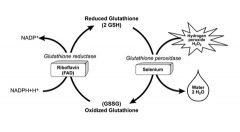
What is the definition of antioxidant?
What are the two antioxiants we have in the mitochondria then? (ONE FOR EACH OXIDANT) |

turns a free radical into something that isn't a free radical
glutathione is an effective free radical scavenger on top of being a peroxidase |
|
|
What is glutathion turned into when it is used up? Give the name and chemical symbol for both.
|
glutathion GSH is turned into glutathione homodimer GSSG
|
|
|
Give the reaction for antioxidant ability of glutathione
|
2OH. + 2GSH → 2H2O + GSSG (glutathione homodimer).
|
|
|
How can you use GSH and GSSG levels to estimate oxidative stress?
|
the more GSSG there is, the more oxidative stress
|
|
|
Give the reaction for antioxidant ability of SOD
|
2O2.- + 2H → H2O2 + O2
|
|
|
Where do we have catalase? Why?
|
In peroxisomes to get rid of excess hydrogen peroxide not used in peroxy (in the name) reactions.
2H2O2 → O2 + 2H2O |
|
|
What are some Endogenous or exogenous antioxidants? (4)
How do they work> |
(e.g., vitamins E, A, and C, and β-carotene) may either block the formation of free radicals or scavenge them once they have forme
|
|
|
What types of molecules reduce ROS formation via the fenton reaction? (4)
|
Anything that binds reduced iron or copper to the blood. (e.g., transferrin, ferritin, lactoferrin, and ceruloplasmin)
|
|
|
Name the three reactions that are particularly relevant to cell injury mediated by free radicals
Summarize what type of damage each results in |
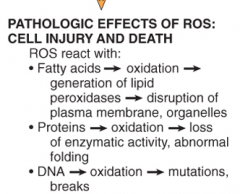
|
|
|
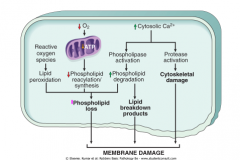
1. Describe the reaction by which free radicals cause damage to lipid cell membranes.
Why does this tend to propogate? |
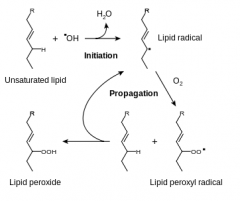
Double bonds in membrane polyunsaturated lipids are vulnerable to attack by oxygen-derived free radicals. The lipid-radical interactions yield peroxides, which are themselves unstable and reactive, and an autocatalytic chain reaction ensues.
|
|
|
Why is it so bad that double bonds are turned into singel bonds?
|
double bonds can't rotate so they used to add to the stability of the membrane. When oxidized, they lose thier functional shape and the membrane becomes more nebulous.
|
|
|
What types of membrane are affected by lpid peroxidation?
Which are the 3 most important? |
all types: lysosome, cell membrane, peroxysomes, rER, nucleus, mitochondria etc
1. mitochondria 2. nucleus 3. lysosome |
|
|
Why is damage to the mitochondrial membrane so deadly?
|
results in decreased production of ATP, culminating in necrosis, and release of proteins that trigger apoptotic death
|
|
|
Why is damage to the plasma membrane so deadly? (2)
|
1. loss of osmotic balance and influx of fluids and ions,
2. loss of other cellular contents such as metabolites that are vital for the reconstitution of ATP, thus further depleting energy stores |
|
|
Why is damage to the lysosomal membrane so deadly?
|
1. leakage of their enzymes into the cytoplasm
2. activation of the acid hydrolases in the acidic intracellular pH of the injured (e.g., ischemic) cell. |
|
|
What types of enzymes are release by lysosomes when they leak? (4)
What process of cell death does this result in? |
Lysosomes contain RNases, DNases, proteases, glucosidases, and other enzymes. Activation of these enzymes leads to enzymatic digestion of cell components, and the cells die by necrosis.
|
|
|
2. Describe the reaction by which free radicals cause damage to proteins
|
Cross-linking of proteins. Free radicals promote sulfhydryl-mediated protein cross-linking
(they can even result in polypeptide fragmentation) |
|
|
What are 3 functions of proteins that would be compromised by cross linking?
there is one that can cause other cells to malfunction as well |
1. enzymatic activity
2. structural integrity (cytoskeleton) 3. signaling activity (leads to damage in other cells. |
|
|
3. Describe the reaction by which free radicals cause damage to DNA
|
Free-radical reactions with thymine in nuclear and mitochondrial DNA produce single-strand breaks.
|
|
|
Is all hell lost when there is a break in DNA? When does all hell become lost?
|
No because there are nick repair mechanisms.
WHen they are overwhelmed, the enzymes will send out a signal for apoptosis however |
|
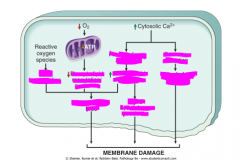
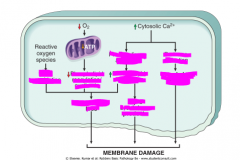
|
|
|
|
What are the ways in which the mitochondrial membrane can be damaged? (3)
|

|
|
|
What are the two outcomes of mitochondrial damage?
|
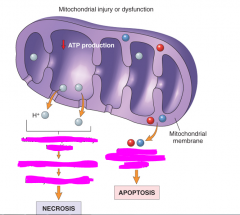
|
|
identify the mechanisms for each outcome
|
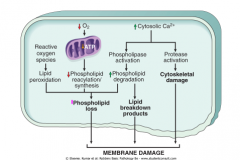
|
|
|
What molecule acts similarly to damaged DNA to trigger apoptosis?
|
misfolded proteins
|
|
|
Let's review the general progression of these previous mechanisms of cell injury.
|
ok!
|
|
|
ATP depletion
|
failure of energy-dependent functions → reversible injury → necrosis
|
|
|
Mitochondrial damage (2)
|
depletion of ATP --> eventually necrosis if prolonged
leakage of apoptotic proteins --> apoptosis |
|
|
Influx of calcium (2)
|
1. activation of enzymes that damage cellular components --> necrosis
2. direct activation of caspases --> apoptosis |
|
|
Accumulation of reactive oxygen species:
|
covalent modification of proteins, lipids, and nucleic acids --> necrosis and apoptosis
|
|
|
Increased permeability of cellular membranes:
|
may affect plasma, lysosomal, and mitochondrial membranes --> necrosis
|
|
|
Accumulation of of damaged DNA and misfolded proteins:
|
triggers apoptosis
|
|
|
Why do we measure troponin?
|
because it leaks out when the membranes get damaged
|
|
|
is necrosis and apoptosis exclusive?
|
no, they can occur simultaneously, especially during ATP depletion --> ROS creation --> cell membrane and content disturbance --> death
|
|
|
What is the AMOUNT of cells that necrosis vs apoptosis refers to?
|
necrosis usually refers to a LARGE group of dying cells and apoptosis a SMALL group
|
|
|
Does running protect from MI? HOW?
|
by forming more collateral vasculature, not by removing plaque
|
|
|
What makes the difference between liquefactive and coagulative necrosis?
|
in coagulative, the tissue architecture is lost
|
|
|
What makes the difference between a pale infarct and a hemorrhagic one?
|
existance of collaterals and whether the tissue is loose
|
|
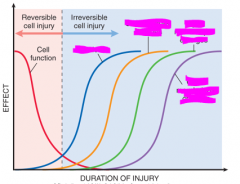
name the order of the events of observing cell death
|
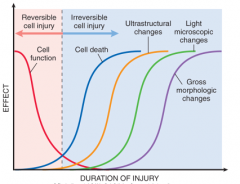
|
|
|
How long does it take cells to lose function without oxygen?
What about to die? What about to be apparently dead by electron microscopy? light microscopy? |
stop functioning- 1-2 minutes
dead- 20 minutes visible by electron microscpy- 2-3 hours visible by light microscopy- 6-12 hours |
|
|
So what window of time do we have reversible cell injury?
|
about 1-20 minutes
|
|
|
What are the two processes that we think tip us over t the point of irreversibility?
|
1. irreversible mitochondrial insufficiency of ATP
2. membrane damage (all 3) |
|
|
Two main morphologic correlates of reversible cell injury?
|
cellular swelling and fatty change
|
|
|
How can you observe cellular swelling grossly and via a microscope?
|
grossly- some pallor, increased turgor, and increase in weight of the organ
microscopic- hydropic change or vacuolar degeneration, cell enlargement, membrane blebbing, loss of microvilli definition |
|
|
What is and what causes hydropic change? Show a comparison pic with a normal cell.
|
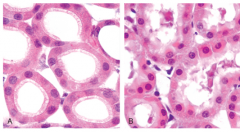
a morphology that develops with cellular swelling- small or large clear vacuoles within the cytoplasm;
|
|
|
closer pic of hydropic change
|
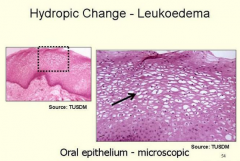
|
|
|
What stains pink and blue? (name each stain and the thing it attaches to)
|
pink- EOSIN STAIN- eosinophilia- proteins (cytoplasmic)
blue- HEMATOXYLIN STAIN- basophilia- RNA (H&E stain) |
|
|
What will a dying cell look like interms of color?
|
progressively more eosinophilic
|
|
|
What are 2 reasons for increased eosinophilia?
|
more denatured proteins out and about - more eosin binding
RNA is degraded - less basophil binding |
|
|
Why does fatty change happen?
What does it look like? WHat is another name for it? What cells does this happen in? |
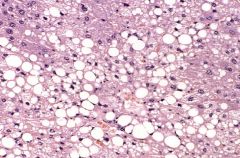
Steaosis- the appearance of lipid vacuoles in the cytoplasm.
happens in cells participating in fat metabolism (e.g., hepatocytes and myocardial cells) |
|
|
show what a swollen and recovering from being swollen cell looks like
|
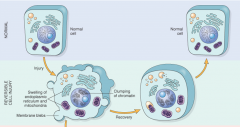
|
|
|
NOW WE TALK ABOUT NECROSIS MORPHOLOGY
would necrotized cells look more homogenous or heterogenous than normal cells? Why? key word? |
"glassy" homogenous because there is no more glycogen (they used it up)
glycogen makes clear vacuoles in the cell on H&E |
|
|
What is the main cause of morphological changes in necrotized cells?
|
degradative action of enzymes on lethally injured cells
|
|
|
Where do these degradative enzymes come from mainly? (2)
|
lysosomes
immune cells to clean up the mess |
|
|
What happens to the appearance of the cytoplasm? Keyword?
Why? |
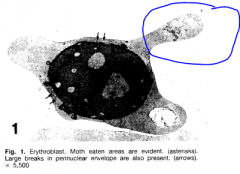
When enzymes have digested the cytoplasmic organelles, the cytoplasm becomes vacuolated and appears MOTHEATEN
|
|
|
wHAT FATTY CHANGES CAN HAPPEN TO NECROTIZED CELLS?
|
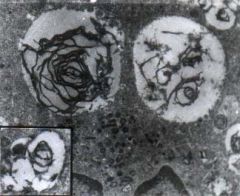
Dead cells may be replaced by large, whorled phospholipid masses, called myelin figures, that are derived from damaged cellular membranes
|
|
|
What are myelin figures made out of?
|
plasma and organelle membranes in lamellar stacks
|
|
|
What is the two fates of these myelin figures?
|
1. phagocytosed by other cells or further degraded into FATTY ACIDS
2. calcification of such fatty acid residues results in the generation of CALCIUM SOAPS. |
|
|
So what is a characteristic change of dead cells that you can pck up on x ray?
|
calcifications
|
|
|
how is the cytoskeleton integrity related to membrane integrity?
|
it holds up the membrane so when it gets degraded, the membrane kind deflates like a tent
|
|
|
What are the 3 possible of nuclear change in necrosis?
|
1. karyolysis
2. pyknosis 3. karyorrhexis |
|
|
define karyo-
|
nucleus
|
|
|
define pykno-
|
thick, dense, compact.
|
|
|
define lysis
|
to break apart
|
|
|
define rhexis
|
bursting or ruture
|
|
|
define karyolysis.
What would you observe on an H&E stain? |

The basophilia of the chromatin may fade (karyolysis), presumably secondary to deoxyribonuclease (DNase) activity
|
|
|
define pyknosis
What would you observe on an H&E stain? |
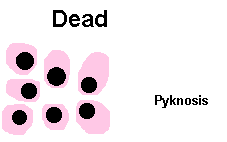
nuclear shrinkage and increased basophilia; the DNA condenses into a solid shrunken mass
|
|
|
define karyorrhexis what stage does it come after?
What would you observe on an H&E stain? |

it is after pyknosis when the condensed nucleic acid fragment.
|
|
|
When is karyorrhexis complete and the nucleus dissolved after cell death?
|
1-2 days
|
|
|
Show an overview comparison of normal vs necrotized celll nuclei
|
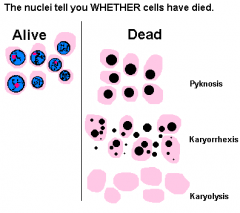
|
|
|
Summarize the hallmarks of morphological reversible cell injury. (5)
|
1. cell swelling
2. fatty change 3. plasma membrane blebbing and loss of micro-villi 4. mitochondrial swelling 5. dilation of the ER 5. eosinophilia (due to decreased cytoplasmic RNA) |
|
|
Summarize the hallmarks of morphological irreversible cell injury. (6)
|
1. increased eosinophilia
3. nuclear shrinkage, fragmentation, and dissolution 4. breakdown of plasma membrane and organellar membranes 5. myelin figures 6. loss of cell content fromleakage and enzymatic digestion |
|
|
Summarize the hallmarks of morphological apoptosis. (2)
|
1. nuclear chromatin condensation
2. formation of apoptotic bodies |
|
|
What are apoptotic bodies? What are they made of?
|
pieces of the cell that bleb off in an organized death
(fragments of nuclei and cytoplasm) |
|
|
NOW WE TALK ABOUT PATTERNS OF TISSUE NECROSIS!
|
YAY!
|
|
|
dO THE ENSUING TERMS reflect underlying mechanisms?
|
NOPE
|
|
|
Why is the protein structure not broken down in coagulatie necrosis?
|
enzymatic processes have altogether stopped including proteases
|
|
|
When is the struture ultimately broken down and by what? How is this done?
|
in a couple days by inflammation by leukocytes that eat them up and digest them in their lysosomes
|
|
|
What does coagulative necrosis look like on microscope before phgocytosis?
|
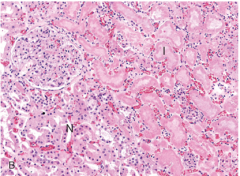
eosinophilic, anucleate cells
|
|
|
What does coagulative necrosis look like on a gross level?
|
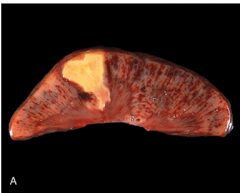
wedge shaped (tibutaries of infarcted vessel) and pale (no blood)
|
|
|
What tissues are affected by coagulative necrosis?
|
all solid organs that undergo ischemia except the brain
|
|
|
What is the main cause of liquefactive necrosis?
What about in the brain? |
inflamation from immune reaction to bacterial or fungal infection
we don't know why the CNS undergoes liquefctive necrosis |
|
|
is liquefactive necrosis usually widesead?
|
no, it is focal
|
|
|
What normal experience is an example of liquefactive necrosis?
|
pus from acne
|
|
|
Why is it so creamy?
|
due to the complete digestion of the fatty cell membrane
|
|
|
When do we call something pus vs liquefactive necrosis?
|
if it was initiated by acute inflammation and probably the result of infection
|
|
|
What is gangenous necrosis? WHere does it usually form and why?
|
it is coagulative necrosis usually in the legs of diabetics who have lost circulation to the legs
It has overlying liquefacive necrosis from the infections these people gain |
|
|
What does caseous necrosis look like?
How is it organized? Are the cells intact? |
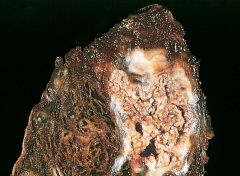
like friable cheese
the cells are lysed, but within definite borders called granulomas |
|
|
Why is the necrosis in defined borders?
|
the affected cells have been walled off by giant cells
|
|
|
Where do you usually see caseous necrosis?
|
in the apex of the lung with tuberculosis
|
|
|
How does fat necrosis appear and why?
What causes it? |
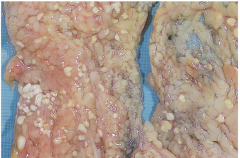
caused by the release of lipases into fatty tissue, resultng in lysis of trglycerides to FA's which are then saponified by calcium producing visible FOCAL chalky white areas
|
|
|
Where does fat necrosis tend to happen and whith what disease?
|
the mesenteric fat due to acute pacreatitis releasing lipases from it's acinar glands
|
|
|
Can trauma cause fat necrosis? example?
|
yes, a punch to the breast can cause fat cell rupture and calcification
|
|
|
What is the pathophysiology of fibrinoid necrosis? What does it look like?
|
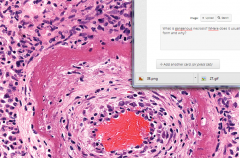
immune complexes deposit in the vessel wall, cause inflmmation, letting fibrin through the endothelial cells to gather beyond them
this appears thick and pink in the vessel |
|
|
Why is it called fibrinoid?
|
because it lets fibrin in
|
|
|
How do AST and ALT get into the blood?
|
they are released by the bile duct and liver when those membranes are damaged
|
|
|
NOW WE TALK ABOUT RESPONSES TO INJURY IN PARTS OF THE CELL (RATHER THAN THE WHOLE)
|
Although some of these alterations occur in acute lethal injury, others are seen in chronic forms of cell injury, and still others are adaptive responses
|
|
|
AUTOPHAGY
Walk me through the process of autophagy (3) |
1. cell is starving
2. a non ribosomal part of the rER reaks off into a autosome and envelops an organelle 3. fuses with a lysosome for degradation |
|
|
How do we know when to start autophagy?
|
there are proteins that sense nutrient deprivation
|
|
|
What happens if the nutrient deprivation continues?
|
apoptosis is triggered
|
|
|
LYSOSOMES
What nutrietns are Lysosomes good and bad at digesting? |
good at protein and carbs
only partially effective for lipids |
|
|
What happens to lysosomes with undigested material? What are they called?
|
they persist in the cell as "residual bodies"
|
|
|
What are some examples of materials that cannot be digested by lysosomes?
|
peroxidized lipids
carbon inhaled from the air ink from tatttoos |
|
|
What happens to residual bodies over time? Specifically what is the result of each of these three substrates?
peroxidized lipids carbon inhaled from the air ink from tatttoos |
they stay there!
peroxidized lipids- lipofuscin pigment granules carbon inhaled from the air- macules in pneumoconiosis ink from tatttoos- your tattoo |
|
|
What are Hereditary lysosomal storage disorders?
|
where a person doesn't have some enzymes to dgest macromolecules in lysosomes and they get an accumulation of residual bodies
|
|
|
What is the function of the sER?
|
metabolism of chemicals
|
|
|
what organ i most likely o undergo hypertrophy of sER and Why?
|
liver because it detoxifies everything as first pass from the gut
|
|
|
What phenomenon does sER hypertrophy explain?
|
tolerance to oral medication over time
|
|
|
is the sER always resposible for detoxification?
explain the exception |
no, sometimes it makes it worse like in the case of CCl4 which is metabolized to ROS's
|
|
|
explain cross tolerance in relation to the sER. Give a specific enzyme in the liver sER.
|
P450 breaks down many things.
Ingesting a lot of one of it's substrates will increase it's capacity to digest another as well. |
|
|
FINALLY MICOTUBULES (LAST ONE)
How do microtubules play a role in adapting the cells increase pressure/mechanimcal stress exxample? |
it remodels itself to better suit the stress demand
example- hypertrophic cardiomyopathy |
|
|
FINALLY MICOTUBULES (LAST ONE)
How do microtubules play a role in cell content transport? example? |
transport- it delivers vesicles and organelles totheir destination
example- chediak higashi inability for platelets to release aggregation factors |
|
|
How do microtubules play a role in cell locomotion? example?
|
it contracts it's actin and myosin to push the cell forward and are also involved in flagella mobility
example- loss of respiratory cilia and spern motility in kartagener's syndrome |
|
|
How do microtubules play a role in cell leukocyte migration and phagocytosis? drug example that inhibits this?
|
same as before, they have the actin and myotin to move the outline of the cell and push it
example- colchicine inhibits microtubule polymerization (formation) so macropahges can get to and phagocytize urate crystals, causing gout attacks |
|
|
How do microtubules play a role in cell division? drug example that uses this mechanism?
|
they form the mitotic spindle and divide the cell
example- vinca alkaloids that bind microtubules and an antiproliferative antitumor effect |
|
|
What happens to the contents of the cell when microtubule asembly and function is interfered with?
|
there will be abnormal accumulation of filaments
|
|
|
MITOCHONDRIA
What adaptations would result in more mitochondria? less? |
MORE- HYPERTROPHY
LESS- ATROPHY |
|
|
What may cause mitochondria to change shape? What is it called when it is abnormally large?
|
megamitochondria- can occur in nutritional deficiencies and alcoholic liver disease for whatever reason
|
|
|
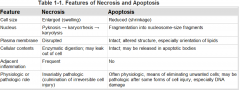
Imagine the difference between necrosis and apoptosis morphology while the cell is dying
|
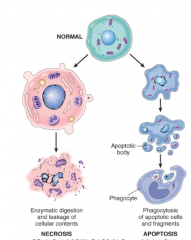
|
|

|
|
|
|
NOW WE DISCUSS APOPTOSIS
Is it pathologic or physiologic |
it is mostly physiologic, but can be pathologic
|
|
|
When is it pathologic?
|
When proteins or DNA are damaged beyond repair
|
|
|
How can a dying cell initiate apopsosis on a healthy neighboring cell
|
lack of growth factor stimulation signalling
|
|
|
compare the ATP role in necrosis vs apoptosis
|
necrosis- due to lack of ATP
apoptosis- very ATP dependent |
|
|
What happens to the apoptotic bodies to clear them? How is this made efficient?
|
they are eated by macrophages nice and neat because they are tagged and tasty
|
|
|
meaning of apoptosis
|
to fall off (the bodies)
|
|
|
does apoptosis induce inflammation? Why?
|
The dead cell is rapidly cleared before its contents have leaked out
|
|
|
What pathology is apoptosis very important in and why?
|
cancer because that's when cells have lost the ability for apoptosis or immune cell mediated apoptosis of fishy cells is not working as well
|
|
|
How can apoptosis dysfunction influence autoimmune disease?
|
you get autoreactive T cells get out into the body
|
|
|
How does apoptosis play a role in our menstrual cycle?
|
hormones induce breast and endomerial cells to grow and then undergo apoptosis throughout your cycle
|
|
|
Why does irreparable DNA damage trigger apoptosis?
|
better kill the cell now before the mutation leads to malignancy
|
|
|
What therapy uses this principle of apoptosis?
|
radiation cancer treatment
|
|
|
What happens to the cell if the injury inducing apoptosis becomes even worse?
|
necrosis
|
|
|
What are two paths by which a cell can undergo apoptosis due to infection?
|
1. induced by the pathogen so that it can spread
2. mediated by T cells |
|
|
What are some examples of pathogens that induce apoptosis?
|
viruses- HIV or adenovirus
|
|
|
What can cause apoptosis in ductal organs? Wat is this called?
|
any obstruction of the duct. pathological atrophy
|
|
|
What does the cytosol and nucleus of a cell undergoing apoptosis look like? Why?
|
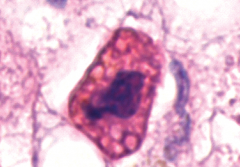
cytoplasm- very eosinophilic from protein breakdown. there are also apoptotic bodies.
nucleus- karyorrhexis from getting the DNA ready to be packaged into proteonucleosomes |
|
|
What is the enzyme that mediates protein degradation in apoptosis?
|
caspases
|
|
|
Why are they called CASPases?
|
they are cysteine proteases that cleave proteins after aspartic residues
|
|
|
How are the nucleic acids broken down then?
|
caspases will eventually activate endonucleases
|
|
|
What are the only two pathways that can induce caspase activation?
|
the mitochondrial pathway and the death receptor pathway
|
|
|
Which method of apoptosis is more used?
|
the mitochondrial pathway
|
|
|
alternate names for the 2 pathways?
|
mito- intrinsic
death receptor- extrinsic |
|
|
Describe the mitochondrial pathway (5 detailed steps)
|
1. some initiating damage, stress, or lack of growth factor
2. a group of many sensor proteins are activated: prototype is bcl-2 family 3. Bcl induces Bax and Bak proteins to dimerize and insert into the mitochondrial membrane 4. This channel allows cytochrome c and antagonists of apoptosis inhibitors will escape 5. cytochrome c activates caspase-9 and other enzymes inhibit apoptosis inhibition APOPTOSIS ENSUES |
|
|
Can apoptosis be reversed after it starts? How?
What proteins do this? (2) WHat is their mechanisms? (2) |
if damage is reversed or growth factor return, the anti-apoptotic members of the Bcl-2 family are activated ( Bcl-2 and Bcl-xL) which inhibit...
1. bak and bax 2. the released pro=apoptotic proteins from the mitochondria that are already out and about |
|
|
Explain the death receptor pathway (5 steps)
|
1. A lymphocyte with a FasL ligand binds with the Fas95/TNF receptor present on almost every cell.
2. binding induces adaptor proteins to cling to the inside of the receptor 3. caspase-8 proteins cluster to bind with the adaptors 4. clustering of caspase 8's will activate them 5. caspase 8's will initiate apoptosis and may also activate another Bcl-2 to initiate the mitochondrial pathway APOPTOSIS ENSUES |
|
|
Is there a way to stop the death receptor pathway? What is the key agent?
How do some viruses take advantage of this? |
FLIP protein is an antagonist of pro-apoptotic proteins downstream of the death receptor pathway
some viruses produce FLIP analogs to escepe being killed by T cells |
|
|
What processes use the death receptor pathway?
|
1. killing autoreactive cells
2. some cytotoxic t cell killings |
|
|
What are some reasons apoptotic bodies are digested so quickly by macrophages?
|
1. the membrane flips in apoptosis so phosphatidyl serine is on the utside attracting macrophages
2. apoptotic cells secrete factors that recruit macrophages to the area |
|
|
Why is it important to quickly eat up the apoptotic cells?
|
left for too long, their membrane would be disrupted
|
|
|
What happens if their membranes become disupted?
|
cell contents leak out and inflammation occurs
|
|
|
With ischemia, does apoptosis or necrosis happen? Be specific!
|
apoptosis happens in early ischemia with some mild damage and necrosis happens later with more extensive damage
|
|
|
Give an example of apoptosis that happens in the brain due to growth factors?
Which pathway does it happen by? |
neurons deprived of neurotrophic factors undergo the mitochondrial pathway of apoptosis
|
|
|
Describe the concept of ER stress and how that happens and leads to apoptosis.
|
1. there is an accumulation of misfolded proteins bcked up in the rER
2. This eventually results in ER stress that activates the caspase system |
|
|
What can this explain about diseases like huntington's, alzheimer's, and parkinson's?
|
they involve misfolded proteins (at least A and H) adn neuronal atrophy. The misfolding probably led to the atrophy!
|
|
|
explain the mechanisms of Cytotoxic T Lymphocyte-Mediated Apoptosis. (1 already talked about and 1 new)
|
1. FasL ligand for the death receptor
2. can insert granzyme through porins that directly activate caspases |
|
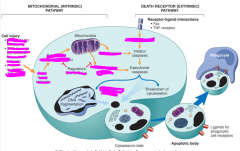
rEVIEW
|
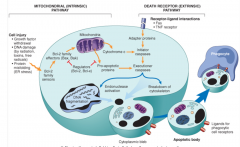
|
|
|
NOW WE TALK ABOUT INTRACELLULAR ACCUMULATIONS
Why is looking at intracellular accumulations useful to assess a disease? |

depending on what you see, you know how far along the disease is (this one is in stage B)
|
|
|
WHat are the 3 categories of substances that can accumulate in a cell?
|
1. a normal substance
2. endogenous substance 3. exogenous substance |
|
|
how do you get accumulation of normal substance?
|
A normal substance is produced at a normal or an increased rate, but the metabolic rate is inadequate to remove it.
|
|
|
give an example of one pathophysiology and one disease that abides by this
|
loss of ability to respond to signals to halt profuction
fatty liver |
|
|
Which steps in the hepatocyte can be affected to explain why there would be steaosis?
Can we pinpoinnt one cause? |
Excess accumulation of triglycerides may result from defects at any step from fatty acid entry to lipoprotein exit, thus accounting for the occurrence of fatty liver after diverse hepatic insults.
|
|
|
How do you get accumulation of endogenous substances? How is this different?
|
the products that accumulate are never made into their final form
|
|
|
What range of steps are affected in endogenous substance accumulation?
Can you give 4 examples of processes? |
anything that goes wrong from DNA to substance packaging and transport
ex.) folding, packaging, transport, secretion |
|
|
What are the two main etiologies of problems with making and secreting a molecule?
|
1. genetic- alzheimer's plaques
2. acquired- creating the wrong protein after radiation exposure |
|
|
So what is the difference between normal substance accumulation and endogenous?
|
normal- all the processes are fine, they are just going at imbalanced rates
endogenous- the processes of the cell don't work right |
|
|
Define exogenous substance accumulation
|
something from outside the body enters the cell and we are unable to remove it fast enough or at all
|
|
|
Give a disease example of exogenous substance accumulation
|
pneumoconiosis- accumulation of coal dust in dust cells (alveolar macrophages)
|
|
|
what is the process of what is done with FA's after it goes into a hepatocyte?
What are the products it is made into and what are they used for? How are they packages and shipped out? |
1. Fa's come in and are converted to triglycerides
2. Fa's are ctabolized to... a. ) membrane components- phospholipids and cholesterol (gives stiff strength) b.) oxidized to ketone bodies for energy or turned into lipoproteins by combining with apoproteins everything is loaded onto lipoproteins to be packaged off to the blood |
|
|
What does CCl4 or lack of proteins do to this whole process? What is the result?
|
it decreases the amount of available apoprotein so that lipoproteins cannot be made and there is accumulation of fat in the liver
|
|
|
How can anoxia lead to steatosis?
|
no oxygen to run the catbolism processes or to oxidize TG into ketones = accumulation
|
|
|
How can alcoholism lead to steatosis?
|
alcohol inhibits mitochondria sER?
|
|
|
what microscopic finding definies foam cells?
|
they have a lot of bubbles of cholesterol inside them
|
|
|
how does this accumulation happen?
|
they eat up too much cholesterol from plaques
|
|
|
What accumulates where in nephrotic syndrome and why?
|
proteins accumulate in the prxoimal tubule cells because they are frantically trying to reabsorb it
|
|
|
Why may you get a dark spot on top of your lymph node?
|
coal filled immune cels migrate to the lymph node
|
|
|
What makes a bruise change color?
|
phagocytes come in to eat the blood and then oxidize the heme so it starts changing different colors
|
|
|
What is lipofuscin also known ask? Why?
|
the wear and tear pigment. It is an accumulation of peroxidized unsaturated lipids that show that there has been previous free radical injury in the celll
|
|
|
What does lipofuscin look like?
|
insoluble brownish-yellow granular intracellular material
|
|
|
What islipofuscin usually indicative of? (2 processes)
|
atrophy and aging
|
|
|
What is it called when there is a lot of lipofuscin in a cell?
|
brown atrophy
|
|
|
Which tissues tend to accumulate lipofuscin the most? Why do you think?
|
heart, brain, and liver
these organs all let ketones into their cells reglarly so are already oxidizing lipids? |
|
|
What color is melanin?
what reaction is it created from? |
brown/black
the conversion of tyrosine to dihydroxyphenylalanine |
|
|
explain the phenomenon of freckles
|
adjacent basal keratinocytes in the skin can accumulate the pigment (e.g., in freckles), as can dermal macrophages
These stay there permanently |
|
|
What is a storage disease?
|
An inherited defect in an enzyme that results in failure to degrade a metabolite.
|
|
|
When is fatty change of the liver irreversible? reversible?
|
when there is accumulation because of an influx of FA, that is reversible.
When there is permanent enzyme damage like CCl4, that is irreversible. |
|
|
So what condition determines wheter intracellular accumulation will be irreversible?
|
when some vital metabolic function is lost
|
|
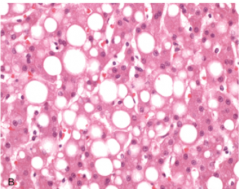
Can you determine what this is from this pic?
|
No, fat, glycogen, and water can all show up as vacuoles in the celll.
|
|
|
How can you prepare the slide to check if it is fat? Stain? resulting color?
|
you freeze it to leave the fat, and then stain with sudan IV or oil red , which will turn fat red-orange
|
|
|
What stain do you use to find out if it is glycogen? resulting color?
|
periodic acid-schiff(PAS) or Best carmine
glycogen is red-violet |
|
|
What if you try both stains and don't get anything?
|
then it is water!
|
|
|
What do early and late fatty changes look like in a cell under the microscope?
|
early- small fatvacuole gather around the nucleus
late- vacuoles expand to shove nucleus to periphery. they may even rupture the cell and coalesce to form fatty cysts |
|
|
What does early and late fatty change look like in the liver grossly?
|
early- barely anything
late- liver can enlarge to become very large and yellow and greasy with fat |
|
|
WHy would the heart get fatty change?
|
hypoxia --> impaired oxidation of fatty acids (main source of energy) --> fatty change
|
|
|
Why would the brain get fatty change then?
|
same reason (hypoxia)
|
|
|
What are two scenarios in which macrophages will take up cholesterol?
|
necrosis and oxidized LDL
encountering lipid debris of necrotic cells or abnormal (e.g., oxidized) forms of lipoproteins |
|
|
What is xanthoma? dissect?
etiology? |
xanth- yellow oma-tumor or mass
it is a mass of subepithelial foam cells made because there was inherited or acquired hyperlipidemia |
|
|
How common are morphological protein accumulations compared to fatty accumulation?
|
not as common
|
|
|
is protein normally absorbed in the prximal tubules?
|
trace amounts of albumin filtered through the glomerulus are normally reabsorbed by pinocytosis in the proximal convoluted tubules
|
|
|
ok back to fatty accumulations for a sec (new book)
What is cholesterolosis? How does it happen? |
This refers to the focal accumulations of cholesterol-laden macrophages in the lamina propria of the gallbladder ( Fig. 1-31 ). The mechanism of accumulation is unknown.
|
|
|
What is the pathopjhysiology Niemann-Pick disease, type-C?
What locations in the body are affected? |
a body-wide cholesterol lysosomal storage disease that is the result of mutation of enzymes trafficking cholesteral
multiple organs, whatever trafficks cholesterol |
|
|
back to proteins.
how can protein accumulations look? (3 types of organization) what do their appearances have in common? WHy? |
1. fibrillary
2. vacuoles/droplets 3. crystalline they are all rounded and eosinophilic because they clump and are made of protein |
|
|
do proteins only accumulate inside the cell? give an example?
|
no, amyloid deposits outside
|
|
|
show and explain russel bodies. what is the protein contained inside of?
|
distended rER filled with immunoglobbulins in plasma cells
|
|
|
what disease are rsussel bodies associated with? Why?
|
multiple myeloma because this is cancer of the plasma cells where they produce too much of their "products" (immunoglobulins and other proteins)
|
|
|
α1-antitrypsin deficiency pathophysiology.
what accumulates where and why? and what disease ensues? |
mutations in the protein significantly slow folding, resulting in the buildup of partially folded intermediates, which aggregate in the ER of the liver and are not secreted. The resultant deficiency of the circulating enzyme causes emphysema
|
|
|
What are the 4 different types ofcytoskeleton proteins?
|
microtubules (20–25 nm in diameter), thin actin filaments (6–8 nm), thick myosin filaments (15 nm) and intermediate filaments (10 nm).
|
|
|
What is the role of intermediate filaments?
|
provide a flexible intracellular scaffold that organizes the cytoplasm and resists forces applied to the cell
|
|
|
What is the role of the others?
|
to move the cell around in phagocytotsis, mitosis, and moving cilia
|
|
|
What are 5 classes of intermediate filaments?
|
1. keratin
2. neurofibrils 3. desmin filaments 4. vimentin filaments 5. glial filaments |
|
|
Which two are especially associated with types of cell injury?
|
keratin and neurofilaments
|
|
|
Which cells are each of these found in?
1. keratin 2. neurofibrils 3. desmin filaments 4. vimentin filaments 5. glial filaments |
1. keratin- epithelial
2. neurofibrils- neurons 3. desmin filaments- muscle 4. vimentin filaments- CT 5. glial filaments- astrocytes |
|
|
what are proteinopathies or protein-aggregation diseases?
|
aggregation of abnormal or misfolded proteins
|
|
|
What does hyaline change mean?
is it just reserved for blood vessels? What types of things can have hyaline change? |
The term hyaline usually refers to an alteration within cells or in the extracellular space that gives a homogeneous, glassy, pink appearance in routine histologic sections stained with hematoxylin and eosin
The previous proteinopathies and ER stress examples count as hyaline change |
|
|
Give me an example of extracellular hyalinization
|
walls of arterioles, especially in the kidney, become hyalinized, resulting from extravasated plasma protein and deposition of basement membrane material.
|
|
|
Why would glycogen accumulation happen? What disease causes it?
What cells are affected? |
too much sugar in the blood from DM causes glycogen accumulation
this goes especially for renal tubular epithelium, B cells of the islets, and heart muscle cells |
|
|
Why renal tubular cells?
|
they are incharge of absorbing glucose back
|
|
|
WHat is it called when there is gycogen accumulation due to a deficit in glycogen metabolism?
|
glycogen storage disease
|
|
|
What is hemosiderin?
|
aggregates of ferritin in a cell with too much iron
|
|
|
how is iron carried around outside and inside the cell? What root do these molecules have in common?
|
outside- carried by transFERin
inside- carried by FERritin |
|
|
What organs would you expect to have a lot of hemosiderin? (3)
What is their common function? |
those that actively break down RBC's
1. spleen 2. liver 3. marrow |
|
|
What are two pathophysiological explanations for hemosiderin formation?
|
1. blood accumulation somewhere to to extravasation
2. systemic overload of iron |
|
|
give two examples of local hemosiderosis.
|
1. bruise
2. ARDS (due to diffuse alveolar damage) |
|
|
How are extravasated RBC's dealt with? How do you know how long this takes?
|
1. macrophages come to eat them up (janitors). they breakdown the heme and save the iron.
several days because that's how long a bruise lasts |
|
|
How do they process the heme?
How can you use knowledge of a bruise to remember the intermediates? |
1. breakdown heme
2. convert heme to biliverdin (green) 3. convert to bilirubin (yellow) this is how a bruise changes color! |
|
|
dissect the word bili-rubin
|
bili- bile
rubin- red "red bile" because it is the final breakdown product of RBC's |
|
|
Which organs get hemosiderin in systemic overload of iron?
|
all of them
|
|
|
What are 3 causes of too mch systemic iron?
|
1. increased dietary iron
2. hemolytic anemia releasing iron 3. blood transfusions introducing new iron |
|
|
What does hemosiderin look like with and without stain?
what stain is used and why? |
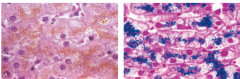
golden without stain
blue with prussian blue which is oxidized by the iron |
|
|
does hemosiderosis normally impair cell functioning? What are two big exceptions?
|
no except in iron transfusional overload and hereditary hemochromatosis
|
|
|
How do you get hemachromatosis?
pathophysiology? |
ingerited. you absorb too much iron.
|
|
|
where is bilirubin normally found?
Sum up what it is. |
in the bile
it is a processed heme without the iron |
|
|
Now we go on to pathological calcification
|
ysy!
|
|
|
what causes dystrophic calcification?
|
necrosis of any kind
|
|
|
where may we see calcifications in a normal aging person?
|
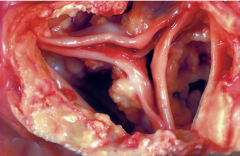
heart valves, atherosclerotic plaques, and the lungs
|
|
|
ho does calcium stain on H&E?
|
basophilic
|
|
|
is there a reason some molecules stain baso vs eosino?
|
they don't have much in common. the dyes just like them better so remember it
|
|
|
What mlecules stain basophilic? (3)
|
nucleic acids and calcium
(and keratohyalin, which is the only protein and is in the skin for keratin) |
|
|
what molecules stain eosinophilic?
|
proteins
|
|
|
What does heterotopic mean? dissect
|
hetero- different
topic- on top of something being somewhere it physiologically shouldn't be |
|
|
What can dystrophic calcification create?
|
heterotopic bone
|
|
|
give an example of heterotopic bone. What does it usually look like and what is a second morphology?
|
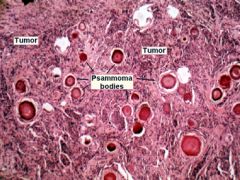
AMORPHOUS- calcium salts have a basophilic, amorphous granular, sometimes clumped appearance.
OR CRYSTALLINE- The progressive acquisition of outer layers may create lamellated configurations, called psammoma bodies because of their resemblance to grains of sand |
|
|
what is the crystal composition of dystrophic calcifications similar to?
what elements are in it? explain how it is formed in necrosis does it stay the same size? Why? |
apatite much like in the bone which contains phosphorus and calcium
1. membrane is disturbed and phopholipases liberate phosphate 2. calcium binds to phosphate and forms a crystal 3. the crystal keeps attracting more calcium and phosphorus and can propogate |
|
|
how is the presene of dystrphic calcifications different thanlipofuscin in a pathological sense? how are they similar?
|
they both indicate past cell damage, but the calcium is more often a further cause of organ dysfunction
|
|
|
give two examples of diseases where it causes further damage by it's growth
|
atherosclerosis
valvular disease |
|
|
What can accentuate dystrophic calcification?
|
hypercalcemia
|
|
|
why do the arteries of old people get brittle?
|
they are atherosclerotic --> inflammation and foam cells burst --> calcification --> hardening
|
|
|
What are the 2 steps to dystrophic calcification?
|
nucleation and propogation
|
|
|
WHat are the 4 main causes of metastatic calcification?
|
1. too much parathyroid
2. destruction of bone 3. too much Vitamin D 4. too much Phosphate |
|
|
What can cause too much PTH?
|
hyperparathyroidism due to parathyroid tumors, and ectopic secretion of PTH-related protein by malignant tumors
|
|
|
What 2 diseases can cause bone destruction and release of caclium?
|
multiple myeloma and leukemia
|
|
|
What 2 diseases can cause too much vitamin D? Exaplin the pathophysiology of each
|
sarcoidosis where macrophages activate a vitamin D precursor
william's disease- infants porn with an abnormal sensitivity to vitamin D |
|
|
Why can renal failue cause hypercalcemia?
|
there is a Ca, PO34- balance. They go up and down together and this is regulated by PTH.
Renal failure --> decrease secretion of phosphate --> increased blood phosphate -- increased match of blood calcium |
|
|
What is milk-alkali syndrome? Dissect the name
|
hypercalcemia due to excessive consumption of calcium like milk or calcium carbonate antaccids
|
|
|
What is aluminum toxicity Why does it happen to?
|
to much aluminum dragging up the calcium as well people on chronic dialysis get this.
|
|
|
Which tissues does metastatic calcification usually affect and why?
|
the tissue that secrete acid and then habe an internal alkali environment that attraccts positive calcium
1. interstitium of gastric mucosa 2. kidneys 3. lungs 4. systemic arteries 5. pulmonary veins |
|
|
how may these calcium deposits appear? compare to dystrophic.
|
they may occur as noncrystalline amorphous deposits or, at other times, as hydroxyapatite crystals.
same options as dystrophic |
|
|
wHAT CAN MASSIVe calcium salts in the kidneys vs the lung do?
|
lungs- restriction of breathing
kidneys- renal failure dir to nephrocalcinoss |
|
|
can we control how much our cells age?
What is the definition of cellular aging? |
yes because cellular agin the the progressive loss of cellular function due to either genetic OR the accumulations of sublethal damage due to exogenous exposures
|
|
|
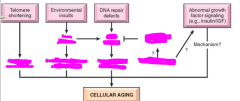
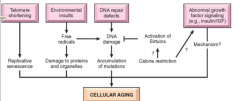
WHat are 4 broad mechanisms that make it so cells can age?
|
1. telomere shortening
2. envrionmental insults 3. DNA repair defects 4. abnormal growth signalling factors |
|
|
|
|
|
define senescence
|
After a fixed number of divisions all somatic cells become arrested in a terminally nondividing state
|
|
|
do senescent cells die off right away?
|
no, they can accumulate
|
|
|
What is wrong with this?
|
then you have a bunch of progressively nonfunctional cells not replaced by better cells
|
|
|
What happens to the capacity of our cells to give off lineages of new cells as we age? In wber's syndrome?
|
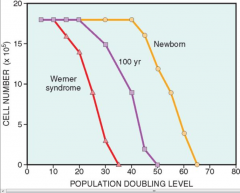
it decreases. In weber's sydrome it is low from the start so those pts age earlier to to more senescent cells
|
|
|
What is a theory on why cells may become senescent?
|
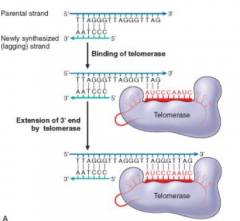
their telomeres shorted after each round of replication because there is no template to copy at the very end
|
|
|
What cells have very high telomerase (2) and very low telomerase?
What cells have no active telomerase? |
high- germ and cancer cells
some- stem cells none- somatic cells |
|
|
WHat are the two ways in which senescence (the process) lleads to accumulation of damaged cells like in againg?
|
1. existing cells become non replicative and accumulate damage
2. stem cells reach their limit and can't replace the old ones |
|
|
Now we talk about Accumulation of metabolic and genetic damage
What is the end result of ROS's on protein, lipid, and nucleic acids? |
covalent modifications of proteins, lipids, and nucleic acids
|
|
|
What mechanism tends to counter environmental damage created by ROS?
|
antioxidants
|
|
|
What happens to this defense system as we age?
|
it goes down
|
|
|
Why are DNA repair mechanisms so important? What could happen if DNA accumulates damage?
|
dna damage --> defective proteins -- accumulaation of protein and loss of cell function --> less replication --> less function --> can't sustain own life --> death
|
|
|
What is the pathophysiology of Werner's syndrome?
|
DNA helicase—a protein involved in DNA replication and repair and other functions requiring DNA unwinding -- is defective and the cell accumulates damage and can't replicate as well.
|
|
|
WHat happens in ataxia-telangiectasia?
|
the DAN that encodes protein that fixes nicks is damaged
|
|
|
On the repair side, what organelle may also be a big contributor to aging as it loses fucntoin? WHy?
|
proteosomes because they are responsible for taking away damaged protiens
|
|
|
WHat is the most effective intervention to prolong the lifespan by increasing repair mechanisms that we have found?
|
calorie restriction
|
|
|
how do we think calorie restriction works?
|
by activating a family of signallling proteins called sirtuins that activate repair mechanisms
|
|
|
WHat enzymatic activity do sirtuins have?
|
histone deacetylase activity,
|
|
|
What is the action of sirtuins?
|
MANY
1. destroy ROS's 2. encourage protein folding 3. increase metabolic activity 4. reduce apoptosis |
|
|
What food may have sirtuin activating factors?
|
red wine!
|
|
|
What signalling molecule can reduce longevity?
how do we know? |
insulin-like growth factor and the things it activates
mutations in it's receptors lead to increased longevity |
|
|
why is it important to have selenium?
|
because it is a vital part of glutathione peroxidase
|
|
|
What does chromatosis mean?
|
nonspecific depositing pigment somewhere
|
|
|
what is siderosis
|
deposition of iron in the body
|
|
|
what is the difference between hemochromatosis and hemosiderosis?
|
chromatosis- excess iron in body
siderosis- excess iron deposited in body |
|
|
what happens to cells that get all their teloere's chppped off?
|
they go into senescence and can no longer replicate.
|

