![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
113 Cards in this Set
- Front
- Back
|
What is the difference b'twn early embryonic mortality, abortion & stillbirth?
|
early embryonic mortality: loss of pregnancy at embryonic stage, before organogenesis
abortion: expulsion of fetus after organogenesis when fetus is not expected to be viable stillbirth: dead fetus delivered w/in period of expected viability |
|
|
What are some mechanisms of abortion?
|
severe maternal illness
-infectious dz causing high fever: mastitis, pneumonia, sepsis -toxins: toxic plants, chemicals (ex. organophosphates) -hypoxia: severe anemia (ex. anaplasmosis), traumatic pericarditis (“hardware dz”: cattle), pneumonia -endotoxemia: Gram (-) infection placentitis -release of PGF-2α --> luteolysis OR -fetal death thru transfer of infectious agents/toxins fetal death: stress initiates premature parturition |
|
|
What are the clinical manifestations of dz of the dam or fetus during:
a. 1st trimester b. 2nd trimester c. 3rd trimester |
a. early embryonic death/resorption
resorption of products of conception: membranes, embryo if calcification started: mummification or abortion regular or irregular return to estrus b. fetal death, abortion, mummification, prolonged gestation c. fetal death abortion fetal maceration &/or emphysema stillbirth prematurity/dysmaturity some neonates survive |
|
|
What is the rate of diagnosis of cause of abortion?
|
~5-50%
of cases in which cause is determined, most are infectious causes (except in equine: more non-infectious causes than other species) |
|
|
What are some common causes of pregnancy loss across species in the following groups
a. bacteria b. protozoa c. viruses |
a. cattle: hematogenous
horses: more likely to infect fetal mems thru cervix -always check placenta in horse to get idea of spread of infection common agents: Brucella, Campylobacter, Chlamydophila abortus, Coxiella burnetti, Leptospira interrogans, Listeria monocytogenes, Mycoplasma, Ureaplasma, Salmonella b. Neospora caninun, Toxoplasma gondii c. herpes virus |
|
|
What are some non-infectious causes of pregnancy loss across species?
|
genetic: tend to be problems that occur before organogenesis
toxins: ponderosa pine (cattle), locoweed, broomweed, Veratrum californicum, nitrate toxicosis (ruminants: methemoglobinemia --> relative hypoxia to fetus), mycotoxins w/ estrogenic activity, chemicals drugs: label warnings, luteolytic drugs, vaccines (particular MLV) nutritional: vitamins & minerals (vit. A def.), body condition environmental: carbon monoxide, weather (heat stress) |
|
|
What are some diagnostic methods for abortion?
|
hx: stage of gestation, age, vaccinations, introduction of new animals, nutrition, recent diseases
specimens -ideal: aborted fetus, fetal membranes, maternal serum, milk, urine, & vaginal d/c -time b’twn infection & abortion is important: autolysis, contamination of specimens w/ environmental agents -fetal mems: 2-3 infected cotyledons or area of infected chorioallantois (fresh, refrigerated, or 10% buffered formalin) -fetus: abomasal contents, lung, liver, kidney, spleen, blood, peritoneal, pleural fluid (fresh or refrigerated), liver, lung, kidney, intestine, other organs w/ lesions (10% buffered formalin) -aborting dam: serum, vaginal d/c, urine -in-contact animals: serum from 10 animals or 10% of herd (consider 2 samples, 10-14 d. apart) serology -single titers in dam rarely diagnostic, except for w/ B. abortus, pseudorabies (regulatory controlled agents) |
|
|
What are the steps of controlling an abortion outbreak?
|
limit exposure of susceptible animals
ID & isolate pregnant animals eliminate source of infection place aborted fetuses in plastic bag/container before moving disinfect environment conduct necropsies away from pregnant animals collect & burn or bury all aborted fetuses & mems isolate domestic animals from area (ex. dogs) collect blood for serology from ~10 or 10% of herd vaccinate susceptible animals if possible be alert to potential zoonotic risk |
|
|
What are some specific causes of abortion in cattle?
|
brucellosis, neosporosis, BVD (time of fetal infection determines outcome), IBR, leptospirosis (5 common serovars; L. interrogans), fungal placentitis, campylobacteriosis (C. fetus ss venerealis), trichomoniasis (Tritrichomonas foetus), listeriosis (L. monocytogenes), EBA, chlamydiosis (C. abortus), ureaplasmosis (U. diversum)
|
|
|
What are some specific causes of abortion in sheep?
|
infectious: enzootic abortion of ewes (EAE: Chlamydophila abortus), campylobacteriosis (C. fetus fetus, C. jejuni), brucellosis (B. ovis), salmonellosis
non-infectious -plant toxins: phytoestrogens, locoweed, broom weed, lupine -stress |
|
|
What are some specific causes of abortion in goats?
|
infectious: chlamydophilosis (C. abortus)
non-infectious -plant toxins: phytoestrogens, locoweed, broom weed, lupine -stress |
|
|
What are some specific causes of abortion in pigs?
|
infectious: PRRS virus, porcine parvovirus (“SMEDI”), pseudorabies, hog cholera (classic swine fever), leptospirosis, brucellosis (B. suis), porcine circovirus 2
environmental |
|
|
What are some specific causes of abortion in horses?
|
infectious: placentitis, equinine rhinopneumonitis (EHV-1), equine viral arteritis, equine mycotic placentitis, other bacterial causes
non-infectious: twinning, endometrial fibrosis (placental insufficiency), premature placental separation, umbilical cord anomalies, mare reproductive loss syndrome, fescue toxicosis |
|
|
What are some specific causes of abortion in dogs?
|
brucellosis (B. canis), herpesvirus, distemper virus, toxoplasmosis, Coxiella burnetti
|
|
|
What are some specific causes of abortion in cats?
|
panleukopenia, feline viral rhinotracheitis, FIP, FIV, toxoplasmosis, Coxiella burnetti
|
|
|
How can abortion be prevented?
|
routinely immunize breeding animals
prevent exposure: institute effective biosecurity, prevent toxin exposure |
|
|
DOG: vaginal edema/prolapse
a. pathogenesis b. 3 types c. causes |
a. exaggerated response to E2 stimulation
-excessive folding of vaginal floor: ventral fold just cranial to urethra that protrudes caudal to urethra -redundant tissue protrudes from vulva b. type I: edema type II: prolapse of ventral fold (pear or tongue shaped; urethra easily seen by lifting prolapsed tissue) type III: entire vaginal circumference (donut shaped) c. constipation, forced separation during coitus, occ. during whelping |
|
|
DOG: vaginal edema/prolapse
a. occurrence b. tx |
a. usually seen on 1st, 2nd, or 3rd proestrus/estrus
-regresses during diestrus -interferes w/ copulation -recurrence is common -breed predisposition: Saint Bernards, English bulldogs, boxers, Dobermans, mastiffs b. differentiate from neoplasia: fibroma, leiomyoma type I: little tx AI in valuable breeding dogs progestagens: early in proestrus GnRH/hCG: little benefit & limited success reported attempt to reposition surgical resection: recommended for type II & III OVH: permanent solution |
|
|
DOG: persistent hymen
a. pathogenesis b. clinical signs c. dx d. tx |
a. failure of Mullerian ducts to unite --> vertical septum (annular fibrous structure)
b. pain on copulation, unable to tie, male refusal to copulate, chronic vaginitis, urine pooling c. digital exam: may find central partition w/ small stoma, single small vaginal opening, R/O normal sphincter contraction vaginoscopy: may miss pathology (can do w/ contrast) endoscopy: useful to evaluate extent of septa d. sx (laser or resection) |
|
|
What are the most common organisms found on vaginal culture in dogs?
|
Pasteurella multocida
Mycoplasma spp. |
|
|
DOG: cystic endometrial hyperplasia
a. pathogenesis b. clinical signs c. dx d. tx |
a. pathologic response to P4 stimulation
estrogen accelerates develops of lesions prolonged P4 --> inflammatory rxn repeated cycles w/ closed cervix --> chronic cystic endometritis predisposes to pyometra b. usually none until pyometra develops c. occasional finding on U/S or during surgical AI uterus may be grossly enlarged no difference in circulating hormones d. OVH |
|
|
DOG: pyometra
a. etiology b. clinical signs c. dx |
a. dogs > 6 yo: predisposed by CEH
administration of exogenous hormones: progestagens & estrogens (mismate shots) canine unique physiology organisms: E. coli overrepresented (>70%) b. vary w/ cervical patency, stage of estrous cycle, bacteria present & duration closed pyometra: depression, anorexia, vaginal d/c, vomiting, PU/PD open pyometra: usually no systemic signs (open cervix); may have irregular cycles PU/PD (E. coli pyometra): pre-renal azotemia, renal damage by glomerular deposition of immune complexes, glomerular dz --> ↓ GFR, ↓ ability to concentrate urine, aggravated by concomitant renal dz c. U/S, rads (may confuse w/ pregnancy if see a fluid filled uterus), leukocytosis |
|
|
What is the tx for pyometra in dogs?
|
OVH: preferred if > 6 yo, not planning to breed anytime soon, or toxemia is present
medical management -hospitalization for at least 3 d. -minimum database: evaluate level of toxemia, P4 assay (should ↓ w/ tx) -systemic ABs -systemic stabilization before uterine therapy -PGF-2α --> luteolysis, myometrial contraction, cervical dilatation -begin w/ 1/10 of doses in handouts: side effects are dose related -sometimes must give PGF-2α for ~2 wks, ABs for up to 30 d. expected results: ↓ uterine size, normal CBC, serial P4 assays to monitor luteolysis, clearer vaginal d/c, improvement in clinical signs subsequent breeding management: important to breed at next estrus; endometrial changes indicate a propensity to develop pyometra |
|
|
DOG: ovarian remnant syndrome
a. what is it b. causes c. clinical presentation d. ddx |
a. clinical signs of estrus in a previously spayed dog or cat d/t retained piece of ovarian tissue: tissue revascularizes & becomes functional
b. surgeon error, accessory ovarian tissue (not common) c. estrus after OVH: mean 9 m, but ranges from 3 m to 5 yrs pseudocyesis (false pregnancy) chronic vaginal d/c: vaginitis d. vaginitis, vaginal neoplasia, uterine stump pyometra, trauma, estrogen therapy |
|
|
How do you diagnose canine ovarian remnant syndrome?
|
difficult to dx
U/S: very specific, questionable sensitivity CBC, Chem, U/A, vaginoscopy presumptive dx: cornified vaginal smear, progesterone assay vaginal smear: esp. useful during supposed “estrus” -if cornification is present: indicates estrogen --> functional ovarian tissue; do serum P4 assay -partial or questionable cornification: swab again in a few days; compare w/ serum P4 results -if no cornification: may be no functional ovarian tissue or animal could be in diestrus or anestrus (compare w/ serum P4 results); presence of vaginal d/c: see ddx P4 assay compare w/ vaginal cytology findings > 3 ng/ml: indicative of functional ovarian tissue 1-3 ng/ml: suggestive of functional ovarian tissue; repeat test in ~1 wk < 1 ng/ml: suggests absence of functional ovarian tissue |
|
|
What is the treatment for canine ovarian remnant syndrome?
|
ex lap
-easier to see during estrus or diestrus (less bleeding during diestrus) -if no obvious functional ovarian tissue: remove scar tissue at both ovarian pedicles: ~100% of remnants at ovarian pedicles; submit tissue for histopath -post-op: patient may develop overt pseudopregnancy: signs subside in < 4 wks hormonal therapy to induce ovulation: helps ↓ signs of estrus -progestagens prevent recurrence: effective, but owners want a permanent solution |
|
|
What are the characteristics of feline ovarian remnant syndrome?
|
similar to dog
time from OVH to estrus: 17 d. to 9 yrs vaginal cytology more informative than serum E2 mating behavior induction of ovulation: GnRH followed by serum P4 assay |
|
|
How would you determine if a female dog is intact or spayed?
|
vaginal cytology, serum P4 assay
-low P4: not in estrus or diestrus, & not pregnant LH assay -high in castrated animals & during estrus -low if not in estrus in intact animals hCG, GnRH stimulation test -run assay for E2 -significant rise in E2 after injection of hCG or GnRH: indicates functional ovarian tissue -no rise: suggests absence of functional ovarian tissue spayed animal -low P4: not in estrus or diestrus, & not pregnant no significant ↑ in E2 post hCG or GnRH: absence of ovarian tissue -LH > 1 ng/ml: lack of ovarian hormones for negative feedback, so hypophysis keeps releasing LH ovarian remnant syndrome -low P4: not in estrus or diestrus, & not pregnant -significant ↑ in E2 post hCG or GnRH: presence of ovarian tissue -LH < 1 ng/ml: suggestive of ovarian hormones causing negative feedback |
|
|
no choke calf snare
|

What is the instrument?
|
|
|
canine obstetrical forceps
|
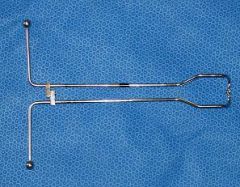
What is the instrument?
|
|
|
detorsion rod
used to assist in the correction of uterine torsion in the cow |

What is the instrument & what is it used for?
|
|
|
Ostertag’s blunt eyehooks
provide an attachment to the head for purposes of traction |

What is this instrument & what is it used for?
|
|
|
fetotomy knives
used to seat the wire for some cuts useful for removing bone fragments prior to fetal extraction |
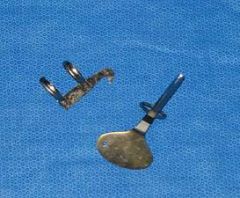
What are these instruments & what are they used for?
|
|
|
Dr. Frank's fetal extractor
|

What is this instrument?
|
|
|
Hercules fetal extractor
|

What is this instrument?
|
|
|
Krey hook
a multiple jointed obstetric implement which approximates its two opposing hooks when these have been anchored in eye-sockets and the end ring is pulled excellent for applying traction to the head of a calf |

What is this instrument & what is it used for?
|
|
|
lamb & kid obstetrical snare
|
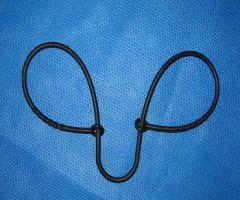
What is this instrument?
|
|
|
modified Utrecht fetotome
double-barreled instrument with a hand grip and notched oval plate for anchoring obstetrical chains |

What is this instrument?
|
|
|
2 types of obstetrical wire handles
|

What are these instruments?
|
|
|
Obstetrical chains & handles
|

What are these instruments?
|
|
|
obstetrical hook
|

What is this instrument?
|
|
|
Obstetrical wire & handles
|
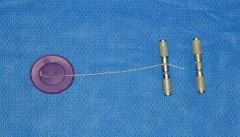
What are these instruments?
|
|
|
plumb bob wire introducer
|

What is this instrument?
|
|
|
porcine obstetrical forceps
|
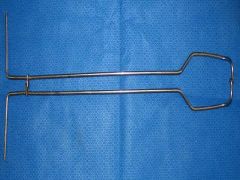
What is this instrument?
|
|
|
used to pass the wire over or around a fetal part
|
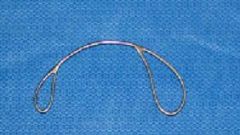
What is this instrument & what is it used for?
|
|
|
Mares are _____ day breeders: season initiated by _________ amounts of sunlight
|
long
increasing |
|
|
What are the 3 main types of estrous cycles demonstrated by mares?
|
seasonally polyestrous: ~85% of mares
-definitive breeding season & periods of anestrus where there is no ovarian activity -enters & leaves breeding season w/ very irregular cycles known as transition periods irregularly polyestrous: 10% -cycle regularly during breeding season & have irregular cycles during transition period, but never really enter anestrus -cycles during winter are very irregular polyestrous: 5% -mares cycle regularly thru out year & seem to be less responsive to light -have periods of anestrus on occasional basis that has no relation to time of year |
|
|
What are the sexual behaviors & cervical characteristics of mares during:
a. proestrus b. estrus c. diestrus |
a. passive behavior during teasing, moderate cervical tone, well define uterine edema (via U/S)
b. estrus: 4-7 d. mare raises tail, squats, urinates frequently in male’s presence, winks soft, edematous pink cervix that droops or lies in floor of vagina most mares ovulate 24-48 hrs. before end of estrus c. diestrus: 14-16 d. mare squeals, pins ears, strikes or kicks when male approaches dry, pale cervix |
|
|
How can seasonality be controlled in mares?
|
artificial lighting schemes have been used to induce cycling in mares: begin ~60-75 d. prior to desired breeding date
-abrupt method: day length extended to 16 hrs -gradual method: day length ↑ by ½ hr per day on a weekly basis by extending light after sunset until 16 hrs of light is reached -intermittent method: day length extended to 10 hrs, followed by 8 hours of darkness, 2 hrs of light, 4 hours of dark |
|
|
How can estrus synchronization be accomplished in mares?
|
altrenogest (Regumate): synthetic oral progestagen
-suppresses signs of estrus & usually prevents ovulation during administration -fed to mares for 10-15 d. & they return to heat 3-5 d. after administration is stopped -tx mare w/ PGF-2α at time of Regumate withdrawal to lyse any CLs present & ensure heat -hCG or GnRH given late in heat to induce ovulation -management program that combines lights w/ progestagens may ↑ efficiency of breeding mares early in breeding season |
|
|
What are some unique features of reproductive anatomy in the mare?
|
relatively short, blunt uterine horns: sag at base of each horn is where embryo usually fixates
very large follicles: 2+ inches in diameter; cystic follicles as occur in cows don’t occur in mares diffuse placentation: many longitudinal endometrial folds help ↑ surface area of uterus for placental contact repro tract suspended dorsally from under lumbar area: ovaries usually above uterus vascular medullary area of ovary is external to cortex & germinal epithelium -hilar area: ovulation fossa -small portion of CL protrudes from fossa, majority of CL w/in interior of ovary; very hard to palpate CL in mare except shortly after ovulation |
|
|
What is the minimum contamination technique for management of mares prone to infection after breeding?
|
wash mare’s perineum & stallion’s penis before breeding
use AI w/ semen incubated in extender containing AB: 80-90% of bacterial CFUs can be killed w/ incubation for 1 hr. if natural service: tx mare’s uterus pre-breeding w/ extender containing AB monitor follicular development closely via transrectal U/S to keep # of breedings to a minimum & as close to but prior to ovulation as possible if mare has hx of retaining fluid in uterus > 1 d. post-breeding: lavage uterus 4-6 h. after breeding w/ saline or LRS, oxytocin (or PGF-2α?) can improve recovery of lavage & help clear seminal debris & inflammatory products +/- systemic ABs: use w/ caution d/t risk of fungal metritis w/ AB overuse |
|
|
HORSE: uterine torsion
a. occurs when in gestation b. dx c. tx |
a. late gestation
b. rectal exam: majority of uterine torsions are cranial to cervix (vs. cow: usually caudal) always consider as a cause of colic in a mare during last trimester of pregnancy c. manual rotation of cervix if mare is at term & torsion < 270º rolling: when diagnosed in last trimester (must anesthetize) standing flank laparotomy: incision on side toward which torsion is rotated ventral midline laparotomy: best approach (provides better access than flank approach) |
|
|
HORSE: pre-pubic tendon rupture
a. predisposing factors b. clinical signs c. tx d. px |
a. draft breed, > 11 yo, poor muscle tone, excessive uterine wt. (ex. twins, hydrops)
b. pronounced ventral edema in mares close to foaling, painful caudal abdomen -blood in milk: very characteristic finding c. if > 330 d.: induce parturition if < 330 d. & incomplete rupture: abdominal support bandages until induction, laxative diet, ↓ fiber bulk d. poor px for repro: attempts to have mare carry a future foal to term are discouraged |
|
|
HORSE: uterine tear
a. causes b. clinical signs c. dx d. tx |
a. most occur 2º to mutation, fetotomy, dystocia, or violent movements during parturition; can also occur in an apparently normal foaling
b. vary from minimal to severe peritonitis c. difficult: palpation of uterus via vagina, rectal palpation, abdominocentesis, transrectal or abdominal U/S d. broad spectrum ABs, oxytocin, supportive therapy, surgical repair in severe cases |
|
|
HORSE: uterine artery rupture
a. signalment b. signs c. dx d. px |
a. seen in older mares w/in 1st 24 hours post-partum
b. hemorrhage may be contained w/in mesometrium (survival possible) or may hemorrhage into abdomen (acute exsanguination usually results) signs: colic, profuse sweating, tachycardia, profound anemia, death c. rectal exam d. poor px for repro in mares that survive |
|
|
HORSE: fescue toxicosis
a. effects of consumption b. toxic principle c. prevention d. tx |
a. prolonged gestation, thickened placenta, agalactica, infertility
b ergot alkaloid that suppresses prolactin release c. remove mares from infected pastures during last 90 d. of gestation d. domperidone (dopamine receptor antagonist: favors prolactin secretion) |
|
|
HORSE: persistent CL
a. what is it b. causes c. tx |
a. CL that fails to regress 14-16 d. post ovulation
b. early embryonic death after maternal recognition of pregnancy has occurred, inadequate PGF-2α c. PGF-2α |
|
|
What are some causes of gonadal hyperplasia in mares?
|
chromosomal abnormalities (XO mares), senile atrophy, exogenous hormone tx (anabolic steroids), equine Cushing’s, congenital
|
|
|
HORSE: anovulatory follicles
a. what are they b. dx c. tx |
a. large (5-15 cm) non-fertile follicles that persist for 1-4 wks: generally spontaneously regress
b. contain echogenic particles on U/S c. if luteinized: can use PGF-2α to short cycle |
|
|
HORSE: luteal insufficiency
a. minimum P4 required to maintain pregnancy b. tx |
documentation on occurrence is lacking
a. 4 ng/ml b. regumate |
|
|
HORSE: granulosa cell tumor
a. dx b. ddx c. tx |
a.
most common ovarian tumor in mare: benign typically unilateral w/ contralateral ovary becoming small & inactive > 85% of cases have ↑ levels of inhibin, which inhibits FSH a. US, inhibin assay b. pregnancy, unusually large ovary w/ multiple follicles c. surgical removal |
|
|
When do stallions reach sexual maturity?
|
~ 5 years
|
|
|
What do the Sertoli cells & Leydig cells produce in the stallion?
|
Sertoli cells: in response to FSH, Sertoli cells elaborate a variety of compounds that are crucial for spermatogenesis
Leydig cells: in response to LH, Leydig (interstitial) cells produce testosterone & estrone sulfate, which maintain feedback mechanisms on ant. pituitary to maintain spermatogenesis, libido & 2º sex characteristics |
|
|
What are some causes & treatments for physical trauma --> infertility in stallions?
|
testes & scrotum
usually d/t breeding accidents if extensive --> testicular atrophy tx: control inflammation, edema, & hematoma formation; ice packs or ice water baths, NSAIDs, broad spectrum ABs, tetanus toxoid penis & prepuce paraphimosis: inability to retract penis d/t preputial injury or dz (balanoposthitis) tx: support penis to ↓ gravitational edema, replace w/in prepuce ASAP psychological trauma: negative experiences assoc. w/ breeding --> abnormal breeding behavior (most can be retrained) |
|
|
HORSE: torsion of spermatic cord
a. signs & tx if < 180 deg. b. signs & tx if > 360 deg. |
a. clinically insignificant
manually rotate before breeding or semen collection b. scrotal swelling & pain usually requires emergency unilateral castration |
|
|
HORSE: hydrocele & hematocele
a. what are they b. dx |
a. hydrocele: accumulation of serous fluid +/- scrotal edema --> dysfunction of spermatogenesis
causes: trauma, neoplasia, idiopathic hematocele: accumulation of hemorrhagic fluid d/t trauma or extension of hemoperitoneum b. U/S to help determine type of fluid: do not puncture! |
|
|
What are some causes of testicular hypoplasia in the stallion?
|
genetic, teratogens, cryptorchidism, infections, toxins, endocrine disturbances, drug administration (anabolic steroids)
|
|
|
What are the most common equine tumors of:
a. testicles b. penis/prepuce |
a. seminoma (uncommon)
b. SCC |
|
|
HORSE: Habronemiasis
a. lesions b. tx |
a. aberrant cutaneous migration of Habronema --> granulomatous lesions (occurs in warm humid climates)
b. ivermectin |
|
|
HORSE: priapism
a. what is it b. possible sequelae |
a. persistent erection in absence of sexual arousal (phenothiazine tranquilizers?)
b. if erection remains for several hrs: penile & preputial swelling --> penile necrosis |
|
|
HORSE: penile paralysis
a. what is it b. causes c. possible sequelae |
a. inability to retract a flaccid penis, 2º to ↓ retractor penis m. tone
b. seen in debilitated or exhausted animals, 2º to myelitis or spinal injury, 2º to phenothiazine tranquilization c. penile swelling --> possible balanoposthitis |
|
|
HORSE: hemospermia
a. what is it b. causes |
a. contamination of ejaculate w/ blood: significant amts. of blood assoc. w/ ↓ fertility
b. urethritis, rents in urethra, penile laceration, cutaneous habronemiasis, inflammation or infection of accessory sex glands |
|
|
What are some infectious causes of infertility in stallions?
|
systemic illness
bacterial infections of genitalia equine viral arteritis coital exanthema (EHV-3) |
|
|
What are some non-infectious causes of infertility in stallions?
|
physical trauma
torsion of spermatic cord hydrocele, hematocele, variocele testicular degeneration & hypoplasia neoplasia Habronemiasis penile paralysis & priapism hemospermia |
|
|
HORSE: bacterial infections of genitalia
a. common organisms involved b. common cause c. dx |
a. Pseudomonas aeruginosa, Klebsiella, Strep zooepidemicus, Taylorella
b. overzealous use of disinfectants on external genitalia c. culture genitalia, ejaculatory fluids |
|
|
How are sheep & goat vs. lama repro anatomy/physiology different?
|
sheep & goats
-spontaneous ovulators -polyestrous, most breeds are seasonally polyestrous -cotyledonary placentation -gestation length: 145 d. lamas -induced ovulators -constant estrus: pretty much breed year round -diffuse placentation -gestation length: 345 d. |
|
|
What are some factors that affect reproduction in small ruminants?
|
season
male effect flushing male: female mating ratio genotype & breeding strategy nutrition & body condition shearing age melatonin |
|
|
What is the male effect in small ruminants?
|
male will induce cyclicity & synchronize estrus of females in transition period (late summer)
isolate male 3-4 wks before introduction to females 1st induced estrus is often silent, followed by early luteal regression (“short” estrous cycle) pre-treatment w/ progestagens or use of teasers --> ↓ early luteal regression ineffective if females are in deep anestrus (mid-summer) or already cycling (fall) |
|
|
What is flushing & what are the results?
|
↑ nutrition quality (↑ metabolizable energy) & quantity in females 3-4 wks before & 3-4 wks into breeding season
--> ↑ ovulation rate, ↑ pregnancy rate, ↓ length of lambing/kidding season most effective when animal’s genetic potential to ovulate is compromised by time of year or poor nutrition |
|
|
What are the goals for the following repro parameters in small ruminants?
a. lambing interval b. length of breeding season c. females cycling at start of breeding d. pregnancy rate e. abortion rate |
a. 3 lambings/2 yrs
b. 45 d. c. 80-95% d. 95% e. < 5% |
|
|
Define the following repro parameters in small ruminants & state the goal ranges.
a. prolificacy b. lambing % c. weaning proportion |
a. prolificacy: avg. # of lambs born per ewe lambing
-goal: 1.5-2.5 b. lambing %: avg. # of ewes lambing per total ewes exposed to ram at breeding -goal: mature ewes > 90-95%, ewe lambs > 75-90% c. weaning proportion: avg. # of lambs weaned per total ewes exposed to ram -goal: at least 1.5 lambs/ewe |
|
|
small ruminant: What are some causes of low prolificacy in males?
|
low BCS, low serving capacity, infertile/subfertile, male:female ratio, seasonal subfertility
|
|
|
small ruminant: What are some causes of low prolificacy in females?
|
low BCS, poor nutrition, post-breeding stress (ex. heat), genotype, inbreeding, phytoestrogens, transitional season breeding, accelerated lambing/kidding, age of flock (young or > 7 yo), high protein/low selenium, early embryonic loss, overfeeding, inadequate synchrony of estrus (AI)
|
|
|
small ruminant: What are some causes of low lambing % in males?
|
low BCS, low serving capacity, infertile/subfertile, male:female ratio, seasonal subfertility, adverse environment, AI errors (technique, handling, quality), dz (foot rot, pizzle rot), inter-ram aggression
|
|
|
small ruminant: What are some causes of low lambing % in females?
|
low prolificacy, low % cycling at mating, high abortion %, phytoestrogens, dz, age (high % in ewe lambs), inadequate induction/synchrony of estrus
|
|
|
What are some causes of low weaning proportion in small ruminants?
|
starvation/hyperthermia/exposure
stillbirth or stillbirth related to dystocia pneumonia abortion other: predation, septicemia, omphalophebitis, white muscle dz, diarrhea, urethral calculi, tetanus, polioencephalomalacia, dock infections, copper toxicity |
|
|
What are the repro effects of consuming phytoestrogens in small ruminants?
|
consuming high estrogen plants (ex. subterranean clovers) -->
-temporary infertility: ↓ ovulation & fertilization rate, ↑ embryonic loss -permanent infertility: prolonged exposure (> 1 mo.) causes uterine changes --> inability to conceive or maintain pregnancy |
|
|
SMALL RUMINANTS: vaginal prolapse
a. incidence b. when it occurs c. predisposing factors d. tx |
a. goal: < 1%
b. last 3 wks of gestation (may occur at time of lambing) c. repro (multiparous prolific ewes, previous dystocia or VP, ↑ litter size), poor forage quality, poor feeding management, low or high BCS, hypocalcemia, pregnancy toxemia, genetic predisposition, etc. d. epidural & ewe saver |
|
|
SMALL RUMINANTS: vaginal rupture
a. cause b. effect |
a. straining assoc. w/ vaginal prolapse
b. death occurs rapidly d/t hemorrhage & shock |
|
|
What is the tx for retained fetal membranes in small ruminants?
|
placenta not passed w/in 12 hrs of delivery
tx: oxytocin q 2 h. until placenta passed, systemic ABs, tetanus prophylaxis |
|
|
SMALL RUMINANTS: hydrometra
a. occurs in which species b. cause c. dx d. tx |
a. does
b. persistent CL for longer than cycle length c. U/S: fluid filled uterus, can’t see fetus ("cloud burst") d. PGF-2α |
|
|
SMALL RUMINANTS: intersex
a. assoc. w/... b. pathogenesis c. highest incidence in... d. prevention |
a. gene for polledness
b. Z gene encodes protein that prevents activation of testis inducing genes -normal males have Sry gene which inactivates Z gene -Z gene related to dominant polled gene -polled homozygous females have no Z protein & are XX sex-reversed females c. dairy breeds of goats: Sannen, Alpine, Toggenburg d. always have 1 horned individual in breeding pair |
|
|
SMALL RUMINANTS: epididymitis
a. common in... b. etiologic agents c. signs d. dx e. tx f. prevention |
a. common in rams, can occur in yearlings
b. etiologic agents: Brucella ovis (mature rams), Actinobacillus spp or Histophilus spp (young virgin rams) c. swelling of epididymis (most commonly tail), rarely systemic signs, low lambing %, or may be asymptomatic d. PE (palpate epididymis for swelling), serology, semen culture: semen sample contains lots of neutrophils e. ABs generally unsuccessful; test & cull all (+) rams f. prevention: cull affected rams, blood test all new rams q 60 d. until 2 negatives |
|
|
What are keys to successful reproduction in cattle?
|
sanitary calving environment
balanced rations effective estrus detection & breeding programs monitor performance & recommend change |
|
|
dairy cows: as milk production increases, conception rate __________. Pregnancy rates: _____
|
decreases
~20% |
|
|
What are the stages of fetal development in cattle?
|
embryonic period: days 0-42
-axis formation, organogenesis -amnion allantois -nutrition to fetus by diffusion in 20-40 d. range fetal period: days 42-calving -fetal growth -chorioallantoic placenta neonatal period: after calving-d. 28 (weaning) -adaptation & growth |
|
|
During what periods of fetal development do losses occur & at what percentage?
|
fertilization failure: 10%
embryonic loss (days 1-42): 10-40% (esp. failure of MRP: d. 4-17) -days 1-7: persistent follicles, poor oocyte & embryo quality, failure of early embryonic development past 16 cell stage -days 8-17: failure of maternal recognition of pregnancy -days 27-42: hormone imbalances: ex. high estrogen, failure of early placental development fetal loss (days 42-calving): 5-25% -failures in placental & fetal development: relatively little is known end result of 1 service: calving rate of ~50% birth – day 28: < 10% -consequence of dystocia, failures of adaptation, calfhood dz, etc. |
|
|
How is pregnancy monitored in cattle?
|
return to estrus: heat markers
progesterone assay (plasma, serum, or milk): high P4 at d. 21: likely pregnant bovine pregnancy specific protein (BPSP) assay: can get false (+) after calving or recent abortion palpation U/S: mainly used in dairy for early detection of pregnancy & sex determination |
|
|
What are considered high risk pregnancies in cattle?
|
male fetuses: larger, more rapid fetal growth
multiple fetuses large fetuses abnormal fetuses -abnormal offspring syndrome: placental problems, prolonged gestation, failure to induce normal parturition *more common w/ in vitro fertilization & cloning *plan for C section |
|
|
What are some management practices to decrease fetal & calf loss?
|
monitor pregnancies, esp. if high risk
follow guidelines for delivery of a calf control of calf gender: sexed semen ($$), transfer of sexed embryos control of # of fetuses in utero: embryo transfer (not widely done), twin reduction (NOT successful: waste of time) |
|
|
What are the goals for the following repro measures in cattle?
a. estrus detection rate b. conception rate for all services c. services per conception d. calving to conception (days open) e. calving interval (days) |
each measure has its problems: consider milk production, nutrition, herd age structure, management goals of farm, climate
distribution, not just mean values are important: what % of cows are w/in goal range? a. 60-70% b. 45-55% c. 1.7-2.2 d. 85-120 e. 365-395 |
|
|
What are the steps in investigating an estrus detection problem in cattle?
|
heat detection is a big problem on dairy farms
records: inter-estrus intervals: ~ 21 d. clinical exam on farm progesterone tests: milk sample taken at time of breeding: P4 should be low if in heat actions: examine semen handling & AI technique, retrain observers/technician, use aids (KAMAR, HEATWATCH), use a no heat detection program if all else fails (Ovsynch) |
|
|
What are some causes of anestrus in cattle?
a. pathologic causes b. physiologic causes |
a. < 10%; ovarian cysts, pyometra, freemartinism, abnormal fetus (mummification), ovarian hypoplasia, ovarian agenesis, segmental aplasia (uterus unicornis)
b. normal pregnancy (most common), postpartum anestrus, lactation/suckling (high milk production), nutrition (low levels of energy) |
|
|
CATTLE: ovarian cysts
a. what are they b. incidence c. clinical signs |
a. anovulatory ovarian structure > 2.5 cm diameter +/- CL
-persists on ovary for up to 40 d. -is a dynamic structure: becomes atretic (not predictable) b. ~ 10%; highest < 60 days in milk (< 60 d. after calving); typically a dairy problem c. anestrus (80%) if chronic: infertility hump: laxity of pelvic ligaments d/t high E2 --> pelvis tilts forward --> hump on top of tailhead |
|
|
CATTLE: ovarian cysts
a. 3 types b. dx c. tx |
a. follicular: thin walled & secrete more E2 than P4
luteal: secrete more P4 cystic CL: normal (occurs in ~1/3 of normal cycling cows) b. can differentiate b’twn cyst types w/ U/S c. GnRH only -hCG only -GnRH, then PGF in 9-10 d.: most common (cycle back more quickly: ~18 d.) -response: 70-80% exhibit heat w/in 24 d. |
|
|
CATTLE: retained fetal membranes
a. incidence b. risk factors c. tx options |
a. < 10%
b. unsanitary calving, dystocia, twins, induced parturition c. manual removal if easy antimicrobials hormones -PGF: probably of little value unless induced parturition -oxytocin: must prime w/ estrogens 1st to upregulate oxytocin receptors in uterus (can’t use estrogens in food animals!) -no tx: monitor |
|
|
CATTLE: postpartum metritis-pyometra complex
a. incidence b. risk factors c. etiologic agents: dairy d. etiologic agent: beef |
a. < 10%; pyometra is important postpartum problem in dairies, but usually a post-breeding dz in beef
b. RFM, 1st lactation cows, unsanitary calving c. usually A. pyogenes (other possible organisms: Bacteriodes, Fusobacterium) d. usually Tritrichomonas foetus |
|
|
CATTLE: postpartum metritis-pyometra complex
a. clinical signs b. tx c. prevention |
a. if pyometra is advanced, CL persists, uterus keeps growing
-clinical sign: anestrus b. metritis: monitor, PGF, antimicrobials (penicillin or ceftiofur) -pyometra: PGF (usually 1 dose is enough) c. good calving environment |
|
|
What are the 4 positive signs of pregnancy in cattle & when during gestation can each be detected?
|
fetal membrane slip (>30-35 d.): chorioallantois slips thru fingers
amniotic vesicle (35-60 d.): spherical, turgid placentomes (> 90 d.) fetus (> 65 d.) |
|
|
Approx. what size is the bovine fetus at each month of months 2-6 of gestation?
|
2 m: mouse
3 m: rat 4 m: small cat 5 m: large cat 6 m: beagle |
|
|
Besides size of fetus, what are some other methods used to estimate stage of gestation in cattle?
|
fetal head (crown to nose) measurement
amniotic vesicle measurement |
|
|
What is the most costly fertility problem in the dairy industry?
|
anestrus
|

