![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
342 Cards in this Set
- Front
- Back
|
Concentration
Amount |
amount/volume
conc X vol |
|
|
Take 1gm of drug and put it into 500ml H2O, what is the final concentration in %, and mg/ml?
|
1gm=1000mg 1000mg/500ml=2mg/ml
= 0.2% |
|
|
Concentrations (NOT PERCENTS)
|
% of 1000= mg/ml
or gm/100ml Example: 2% Lidocaine % of 1000 = mg/ml 2% of 1000= 20mg/ml EX 2 0.2% = 2mg/ml 0.75% bupivicaine = 7.5mg/ml Lidocaine 1% = 10mg/ml Bupivicaine 0.75 = 7.5mg/ml |
|
|
How many mg are in 5ml of 4% cocaine solution?
|
Amount = conc x vol
40 X 5ml= 200mg |
|
|
Concentrations
|
Epi
1:100,000 or 10mcg/ml 1:200,000 or 5mcg/ml (common conc) 1:500,000 or 2mcg/ml |
|
|
Epi labeled in gm/ml
want to know mcg/ml |
1:200,000 or 1gm in 200,000ml
if 1gm = 1000mg or 1,000,000 mcg 1,000,000/200,000ml = 5mcg/ml Same holds true for epi 1:100,000 1gm in 100,000ml 1gm = 1000mg or 1,000,000mcg 1,000,000/100,000=10mcg/ml |
|
|
Calculating the % of conc to mg/ml
A 25 ml vial of 1% lidocaine with 1:500,000 epi contains how many mg of each drug? |
As before, lidocaine is 1% or 10mg/ml
total amount= conc x vol 10mg/ml x 25ml= 250mg of lido epi 1:500,000 = 1,000,000/500,000=2mcg/ml 2mcg = .002mg .002mg/ml x 25ml= 0.05 mg of epi |
|
|
E cylinder pressures
If flow of O2 from the E tank is 5 l/min, how long until empty? |
@ 20C full E tank has 2200 PSI and 660L.
660L/5L/min = 132 min or 2hrs and 12min |
|
|
A half full cylinder that flows at 10 l/min?
|
@ 20C, half full e tank would have 1100psi and 330L, so
1100/330 = 33min |
|
|
LBS to kg
|
divde by two and subtract first number from total, or first two if 100 or greater
ex 173lbs = 86.5 - 8 = 78.6kg 400lbs = 200lbs - 20 = 180kgs (180kgs x 2.2 = 396lbs, so close) |
|
|
Which unit is associated with this commonly used ratio 1:1000.
|
gm/ml
|
|
|
An epinephrine solution of 1:100,000 equals _______mcg/ml
|
10
1: 100,000 = 1 gm/ 100,000 ml 1 gm = 1,000,000 mcg 1,000,000 mcg/ 100,000 ml = 10 mcg/ml |
|
|
An epinephrine solution of 1:1000 equals ______ mcg/ml
|
1000
1: 1000 = 1gm/ 1000 ml 1 gm = 1,000,000 mcg 1,000,000 mcg/ 1000 ml = 1,000 mcg/ml |
|
|
A 2% solution of lidocaine contains _______ mg/ml
|
20
% of 1,000 = mg/ml 2 % of 1,000 = 20 mg/ ml |
|
|
You gave 20 mls of 0.5% bupivacaine. How many mg did you give?
|
100mg
% of 1,000 = mg/ml 0.5 % of 1,000 = 5 mg/ml 5 mg/ml X 20 ml = 100 mg |
|
|
You are giving a spinal block for C-section. The book suggests mixing fentanyl and bupivacaine together for this block. The book recommends the following dosage: Bupivacaine 12 mg along with 25 mcg of fentanyl. The spinal tray comes with 0.75% bupivacaine and fentanyl comes in 2 ml vials of 50 mcg/ml. Calculate the following:
|
a. How many ml of bupivacaine will you give?
7.5% bupivicaine = 7.5mg/ml 12mg/7.5mg/ml = 1.6ml b.How many ml of fentanyl will you give? 50mcg/ml / 25mcg = 0.5ml c.What is the concentration of bupivacaine in the final mixture? Total volume is now 2.1ml, so 12mg/2.1ml= 5.7mg/ml d.What is the final concentration of fentanyl in the mixture? 25mcg/2.1ml = 11.9mcg/ml e.How many total ml will you be giving? 2.1ml |
|
|
You are pre-oxygenating your patient prior to induction of a general anesthesia. You are giving the patient 8 liters of O2 through the mask on the anesthesia circuit. Answer the following:
a.You are providing _____% oxygen. b.The vapor pressure of oxygen in this example is _________. |
100%
760mmHg |
|
|
You are providing regional anesthesia for a hip replacement surgery. You need to give 12mg of 1% tetracaine with epinephrine 1:200,000
a.How many total ml's of tetracaine will you give? b.How many total ml's of epinephrine will you give? |
1% tetra = 10mg/ml
12mg / 10mg/ml = 1.2ml of tetra Epi 1:200,000, 1gm in 200,000 1gm = 1,000,000mcg conc = amount/vol conc = 1,000,000 / 200,000 = 5mcg/ml 1.2ml x 5mcg/ml = 6mcg or 0.006ml |
|
|
You are providing general anesthesia. You machine settings are as follows:
Oxygen 1.5 LPM , N2O 1.5 LPM, isoflurane 1.2%. Calculate the following: a.Partial pressure of oxygen. b.Partial pressure of isoflurane. c.Partial pressure of N2O. |
Iso = 1.2%
100% - 1.2% is 98.8% since both O2 and N2O are at the same rate, thee is a 50/50 mix or 98.8% / 2 = 49.4% each 1.2% x 760mmHg = 9.12mmHg 49.4% x 760mmHg = 375.44mmHg for O2 and N2O |
|
|
Atmospheric pressure
|
760mmHg at sea level
changes with altitude |
|
|
1 atm
|
760mmHg
|
|
|
Nitrogen
Oxygen |
79% of 1 atm
21% of 1 atm |
|
|
Vapor pressure @20C
|
Sevo 170mmHg
Enflurane 175mmHg Iso 239 mmHg Halo 243 mmhg Des 669 mmHg |
|
|
Equations
|
K = C + 273.16
C = K - 273 C to F = C(9/5 or 1.8) + 32 F to C = (F - 32)/1.8 |
|
|
Critical numbers
|
35-39 centigrade
37.5 centigrade normal |
|
|
Boiling points
|
373.15K
100C 212F |
|
|
Freezing points
|
273.15K
0C 32F |
|
|
165lbs to kg
|
165/2 = 82.5
82.5 - 8 = 74.5kg |
|
|
Capnography
|
Predicts PCO2
Identifies changes r/t patients physiological status helps to identify equip probs ET tube placement Malignant hyperthermia |
|
|
How does it work?
|
control gas sample of CO2 is compared to sample form breathing circuit
IR lights @ 2 wavelengths (2600 and 4300 nm) Calculation displayed as a number/waveform |
|
|
What is the gold standard for ETT verification?
|
Capnography
|
|
|
Where does the calculated number usually run?
|
5-10 less than arterial PaCo2, so...
normal is 35-45mmHg, then try to stay around 32-33 (range could be anywhere from 25-40) |
|
|
A variance in wave form is good for what?
|
Diagnosing many resp probsranging from mechanical to physiological
|
|
|
Should the CRNA always pay close attention to the wave form?
|
Yes
|
|
|
How long should the sample line be? Are debris/condensation/kinks okay?
|
Sample line should be as short as possible, and clean of debris/condensation, with no kinks. The filter must be clean as well
|
|
|
Capnograms
|
see http://www.capnography.com/new/index.php?option=com_content&view=article&id=95&Itemid=61
|
|
|
Pulse oximetry
|
detects early signs of hypoxemia
Accurately predicts PaO2 (Press of O2 in blood) Accurately detects Sao2 (Sat Hgb) |
|
|
Pulse Ox is based on whose law?
|
Beers
|
|
|
Beers law
|
I transmitted = (I incident)(e-DCa),
where D is distance into the solution, C is the concentration of the sub in liquid, a is a constant |
|
|
How does it work?
|
Beers law, basically the intensity of the light is altered as it travels through the liquid. the intensity of the light falls exponentially
|
|
|
How many wavelengths are there?
How do the travel? |
2 wavelengths, 660nm (red), and 940 or 990 IR are passed through the arterial bed in a pulsatile fashion
|
|
|
How are the waves analyzed?
|
based on the light reaching the detector
|
|
|
Is a good arterial bed necessary?
|
It is very important, so yes
|
|
|
Does pulse ox respond to non pulsatile signals?
|
No
|
|
|
Why use IR and red light?
|
Red light is absorbed by deoxyhemoglobin, Ir is absorbed by oxyhemoglobin
|
|
|
How is SaO2 calculated then?
|
Based on a preprogrammed calibration curve which is based on beers law.
|
|
|
When will pulse ox not work?
|
not plugged in or turned on (look for light)
Applied to a thick skin area (thumb) Patient is moving Cautery Non pulsatile area, low rate of perfusion form vasoconstriction, hypothermia, hypotension Methylene blue Very anemic patient With certain room lights Methemoglobin (impairs unloading) CO poisioning (abnormal high reading) fake nails |
|
|
Where to put the sensor
|
Fingers (index)
Ears (right ear most accurate of all places) Toes Wherever you can get it to work |
|
|
If you question the result/reading, what should you do?
|
Check the waveform
|
|
|
Can you mute the alarm?
|
NO, always have the sound turned on.
|
|
|
Agent analyzers
|
Mass spectrometry
Infrared technology Non dispersive infrared (NDIR) Dispersive infrared (DIR) |
|
|
Mass spectrometry
|
Compares mass to charge ratio
3 parts Ion source Mass analyzer Detector system |
|
|
Which law of motion applies to the mass spec
|
Newtons second law of motion
Acceleration of the particle is inversely proportjional to the mass Momentum is the product of mass and velocity |
|
|
Do ions with different weights act differently?
|
Yes
|
|
|
What happen as the charged particles move through the magnet?
|
Heavier particles are pulled less than the lighter ones when moving at the same speed.
|
|
|
Physics of sector mass analysis
|
Need an ionized gas
Accelerate the ions to high speed Ions pass throughthe magnet(ic) field (Mag field is perpendicular to the plane of ion flow) |
|
|
Time of flight mass analyzer
|
Electrical field accelerates ions (applies same kinetic energy to each particle)
Ions pass through same potential field Measures the time it takes for ions to reach detector Velocity is dependent on KE and M |
|
|
NDIR, Non dispersive infrared
|
Commonly used with anesthesia ventialtors
Identify a specific gas/agent |
|
|
Keys to proper use/function of NDIR
|
must be turned on to the IR absorbtion characteristcs of the individual gas
ltd by number of filters and detectors an anaylzer contains must e preset with the characteristics of each gas. |
|
|
DIR, Dispersive infrared
|
New technology with single filter and a detector with a diffraction grating which separates different wavelenghts for each agent
|
|
|
Advantages to DIR
|
Fast warm up time
Alcohol and acetone detection Dual agent measurement (important) |
|
|
Why is the agent analyzer really used?
|
To detect amounts of the agent, N2O, and O2, although FiO2, FaO2, and CO2 can also be measured
|
|
|
Nerve stimulators (types)
|
Adjustable or Nonadjustable stimulator for monitoring neuromuscular blockade
Electrical stimulators for regional anesthesia |
|
|
Anatomy of the NMJ
|
Impulse transmittd down the motor nerve, ACH released at the NMJ, ACH attaches to the motor end plate causing myo cell contraction
|
|
|
Classifications of neuromuscular blockers
|
Depolarizing/Non depolarizing
|
|
|
Depolarizing
|
Succinylcholine, causes contractions
|
|
|
Non depolarizing
|
all others; they vary in onset and duration, no contraction
|
|
|
Depolarizing NMB, Succs
|
Acts like ACH
Attaches to motor end plate and causes depolarization Rapid onset and short acting |
|
|
Non depolarizing NMB
|
Attaches to same receptor as ACH,
however blocks the action of ACH, no muscle contraction. |
|
|
Function of nerve stimulators
|
Assess the degree/type of block
Locate nerve branches for regional blocks |
|
|
When do you paralyze a patient?
How much paralytic do you normally give? |
Abd case, knee/tendon repairs
Varies, don't really know how much to give |
|
|
When can you give a reversal agent?
|
When the patient has begun to reverse on there own
|
|
|
What does the nerve stimulator do?
|
supplies electrons to depolarize the nerve
|
|
|
What is the number of electrons per stimulus called?
|
current
|
|
|
What is a muscle contraction called?
How is the strength of the contraction measured? |
Twitch
Twitch height |
|
|
What part of the nerve is polarized?
|
outside
|
|
|
What will adding a negative charge to the membrane do?
|
neutralize the membrane
|
|
|
What is the purpose of neutralizing the membrane?
|
Depolarization
Sends impulse to motor end plate |
|
|
Where do you place the negative electrode?
|
Place the negative electrode a near as possible to the nerve you are stimulating. (Black lead needs to be over nerve, red can be anywhere)
|
|
|
What nerves are commonly use for stimulation?
|
Ulnar (more closely r/t diaphragm)
See movement of thumb Adductor pollicis Facial (faster, more accurate) Movement of eyelid Orbicularis oculi Post tibial Assess toes |
|
|
Types of stimulation
|
Single twitch
Train of four Tetany Double burst stimlation |
|
|
Single twitch
|
Fixed current for a brief second
0.1-1 Hz for .02 sec Often used prior to giving muscle relaxant |
|
|
Why and when is single twitch best to use?
|
Provides control, you can set it to repeat every so many seconds (lets you know when you can intubate)
Best for induction, intubation, works well with non depol NOT SUCCS!!! |
|
|
Train of four
|
4 twitches at 1/2 sec intervals
|
|
|
Which twitch is your control twitch?
|
1st one
|
|
|
With NDMR, what occurs?
|
2-4 usually decrease in amplitude
ACH is depleted with each stimulation Wait >30 sec between testing |
|
|
How are the twitches documented?
|
as x/4 or IIII
EX: 1/4 or IIii |
|
|
When is the patient ready for intubation?
|
@ 1/4 or I
|
|
|
When do you see fade?
|
with NDMR, will look like IIIi, IIii, Iiii
|
|
|
Train of four after DMR
|
Immediately after admin may be no response to nerve stimulation
|
|
|
What might you see after a few minutes?
|
Low intensity, consistent TOF, intensity will gradually increase until returning to pre-induction action
|
|
|
With DMR, will there ever be a loss of all four twitches?
|
No, they will always bee seen, however the amplituted changes
IIII iiii iiii IIII |
|
|
What is Tetany? Why is it used?
|
Sustained stimulation (1 second)
50-100Hz (small sustained stimulation) Used to measure fade |
|
|
What is post tetanic stimulation?
|
Used to determine the depth of the block when you have a 0/4 TOF
|
|
|
Three steps of post tetanic stimulation
|
1. Sustained tetany (50Hz for 5 sec)-loads MEP with ACH
2. wait three secs 3. series of single twitches |
|
|
How many twitches should be elicited when using the method of post tetanic stimulation?
|
no more than 10 twitches should be elicited, at ten twitches your are at approximately a 1/4 TOF
|
|
|
Double burst stimulation
|
two twitches seppararted by breif interval
Easier to detect fade Used at the end of the case to to determine strength II ii II ii |
|
|
What do the nubers with the TOF mean?
|
1/4= 90% blocked
2/4= 80% blocked 3/4=75% blocked 4/4=<70% blocked |
|
|
Why do we reverse patients?
|
To give them back control of their muscles so that thy can help with breathing
|
|
|
What are the two phases of blocks?
|
Phase I, depolarizing
Phase II, nondepolarizing |
|
|
Phase I block
|
Depolarizing
|
|
|
Does fasiculation precede the block?
|
Yes
|
|
|
Is there fade with phase I
|
No, no fade with succs
|
|
|
TOF, Post tet facilitation, ration with phase I block
|
TOF = decreased amplitude without fade
No post tet facilitation Train of 4 ration >.07 |
|
|
Phase II block
|
non-depolarizing
|
|
|
Are muscle faciculations seen with Phase II blocks?
|
no
|
|
|
Is there a fade response with phase II in regards to tetany and TOF? Is post tetanic facilitation available?
|
Yes, fade is present for both, post tetanic facilitation is available
|
|
|
Can you get a Phase II block from succs? Is this good or bad?
|
You can get a phase II block form succs, this is very bad. This type of block can last 2-6 hours and is created form big dose admin or repeated doses with no twitches
Key here is to check twitched between doses as succs |
|
|
How does succs create a phase II block?
|
Blocks the muscle cells ability to repolarize
|
|
|
Emergence
|
need at least one twitch befrores can give reversal agent
|
|
|
What medication is used for reversal of NMBs?
|
Neostigmine
|
|
|
How does Neostigmine work?
|
blocks the acetylcholinesterase, allows for ACH to build back up in the NMJ
|
|
|
If a patient has no twitches, is it safe to give neostigmine?
|
No
|
|
|
How do locals anesthetic work?
|
Stop the movement of sodium
|
|
|
Nerve stimulators for regional anesthesia are used for?
|
locating nerves
|
|
|
Is the amplitude fixed or adjustable? What is the range?
|
Adjustable amplitude, 0-5mA
|
|
|
How many leads are present?
|
2
|
|
|
Where are the leads attached?
|
Negative is attached to the skin, positive to the insulated needle
|
|
|
What is the target goal for maximal stimulation?
|
0.5mA
|
|
|
When is it safe to inject the regional anesthetic?
|
0.2mA
|
|
|
atomic number
|
number of protons in the nucleus of an atom
|
|
|
Element
|
matter composed of atoms that all have the same atomic numbers
|
|
|
Atom
|
smallest unit of an element to retain all the chemical properties of that element
|
|
|
Subatomic particles
|
electrons, protons, neutrons
|
|
|
Organic Chemistry
|
came from word organism
Historically, believed that all organic compounds came from living organisms theroy known as vitalism Disproved in 1828, urea synthesized from inorg comp |
|
|
Atom
|
Carbon is the key compund
Organic chemistry is the study of carbon and its compounds |
|
|
Organic molecules are classified how?
Why? |
Functional groups, tell us how they are going to react
|
|
|
How many carbon compunds are there?
|
More than all other compounds combined
|
|
|
Why is there a greater number of carbon compounds?
|
Carbon can from strong covalent bonds to each other
carbon can form strong bonds with atoms of other non metals |
|
|
Atomic weight
|
number of protons + neutrons
|
|
|
What distinguishes the characteristics of an atom?
|
Number of protons and electrons
|
|
|
Isotopes
|
Atoms with same number of protons and electrons, with different number of neutrons, have smae characteristics with different atomic weight
|
|
|
Electrons have what charge?
How is the arrangement in orbit set up? |
negative
2 electrons in the 1st, 8 in each additional |
|
|
How do atoms attempt to fill their shell with 8 electrons?
|
may give up an electron, attempt to gain one, or share
|
|
|
What is the purpose of a bond between molecules?
|
holds them together
|
|
|
What is it that causes bond to form?
|
Opposite charges attrac, the + nucleus of one atom attracts the negative charged electrons of another atom (this attraction is inversely proportional to the distance between the center charge).
|
|
|
Do electrons gather up next to one another?
|
No, they spread out in space, they repel each other due to like charges
|
|
|
What happens when a bond is formed?
|
reactions occur
|
|
|
Are atoms created or destroyed when chemical reactions occur?
|
neither
|
|
|
WHat is an exothermic reaction? endothermic?
|
exothermic = releases heat
endothermic = absorbs heat |
|
|
Ionic bond
|
complete transfer of an electron
requires energy must be favorable, requires a great deal of energy transfer of the electron forms a cation + and an anion - |
|
|
What is an example of an ionic bond?
|
NaCl (Simple salts)
Na 1+ in outer shell, one ee in outer shell Cl 1- in outer shell, 7 ee in outer shell Atoms with a significantly different electronegativity Na 0.93 Cl 3.16 Creates a full ionized (charged) compound) |
|
|
What is a covalent bond?
|
sharing of electrons
|
|
|
Does a covalent bond require more or less energy than ionic bonds?
|
Less, example HCL
|
|
|
Are covalent bonds symetrical or polarized?
|
either, depending on the electronegativity
|
|
|
What is symmetric covalent bond? How many molecules form complete symmetrical bonds?
|
Net neutral charge
very few |
|
|
What does it mean by polarization?
Give an example? |
there is a charge present
Example: carbon (2.55) and chloride (3.96) electronegativity difference of 1.4, thus polarization |
|
|
What is the purpose behind polarization?
|
Allows simple structural units to form more complex molecules
|
|
|
How does a covalent bond work?
|
sharing of electrons by overlap of the individual atomic orbitals
electrons in each atom tend to become attracted to the positive nucleus of the other atom. |
|
|
How does a hydrogen bond work?
|
electrons in the 1s atomic orbital are attracted to the positive nucleus of the other atom.
The atoms will continue to come together until the nuclei begin to repel each other. The repulsive and attractive forces balance, defiones the bond distance. |
|
|
What is a valence? What are some examples?
|
the number of atoms that are typically bonded to a given atom
Hydrogen - 1 Oxygen - 2 Carbon - 4 |
|
|
What is the name of the simplest hydrocarbon?
|
methane, CH4
|
|
|
What is methane's most stable configuration?
|
Tetrahedral, looks like a pyramid with the nucleus in the middle and the electrons at the corners (only three corners).
|
|
|
When they say something is halogenated, what does this mean?
|
Makes less flammable
|
|
|
What is ethane?
|
2 carbon atoms, C2H6
|
|
|
Hybridization
|
is the mixing of atomic orbitals to form new orbitals suitable for bonding.
Develop different characteristics Example: Methane The 2p and 2s orbitals join together and make up the sp3 orbital. |
|
|
What are some characteristics of carbon, how many bonds can it form?
|
4 electrons in outer shell
Form strongest covalent bonds in nature Single, double, and triple bonds |
|
|
What is an alkane?
|
simple hydrocarbon, composed of carbon and hydrogen only
can vary in number of molecules and existing bonds between these molecules |
|
|
Alkane chain prefixes/number of carbons
|
Meth 1
Eth 2 Prop 3 But 4 Pent 5 Hex 6 Hept 7 Oct 8 Non 9 Dec 10 |
|
|
What is an alkyl group?
|
CH3, a hydrogen on the min chain is replaced by an alkyl group
|
|
|
4 carbon attachment terms
|
Primary- attached to one carbon
Secondary-to two carbons Tertiary-to three carbons Quaternary- to four alkyl groups Quats do not pass the BBB as easily |
|
|
Nomenclature
|
Hydrocarbons having no double or triple bond functional groups are classified a alkanes or cycloalkanes (rings).
Aromatic rings are not included as the contain double bonds. |
|
|
Nomenclature cont
|
First define the longest carbon chain
identify/name groups attached to the chain number chain consecutively, start at the end nearest the substituent group designate location of substituent group by number/name Assemble name in alphabetical order |
|
|
What are some simple ethyl groups?
|
CH3- methyl
C2H5- ethyl CH3CH2CH2- propyl (CH3)2CH- isopropyl |
|
|
What would 2 methylbutane look like?
|
CH3CHCH2CH3
I CH3 |
|
|
How about 2,2,5-3 trimethylhexane
|
CH3
I CH3CCH2CH2CHCH3 I I CH3 CH3 |
|
|
What happens when hydrocarbons are saturated?
|
a single bond exists between all carbon atoms, leading to saturation.
H I H-C-H I H |
|
|
Alkenes/Alkynes-saturated or unsaturated?
|
Both are unsaturated, as either a double bond (kenes) or triple bond (kynes) exists between the carbon atoms.
unsaturated = loose H(s) to form double or triple bond |
|
|
Cyclic compunds
|
C6H6, aromatic rings with double bonds
|
|
|
What is an isomer?
|
Organic compound with similar chemical formula but different structure
Same atomic weight, different physical characteristics |
|
|
Where can an isomer occur?
|
in organic compounds that have more that 3 carbon atoms
|
|
|
What is a conformational isomer?
|
a isomer that exists in different 3D formations
|
|
|
What is a steroisomer?
|
attached in the same sequence but different spatial arrangement
cis=same side trans=opposite side optical isomer (see later card) |
|
|
Enantiomers are...
|
mirror image configurations of two molecules that are not superimposeable on one another
|
|
|
Diastomers are...
|
not mirror images of one another
|
|
|
What is an optical isomer?
|
2 types:
Dextro-isomer (d-isomer) (R-rectus) Levo-isomer (l-isomer) (S-sinister) It is an enantiomer, or mirror image Both forms are isomers in the same compound |
|
|
What is a racemic isomer?
|
equal amounts of the isomers are mixed in the same compound
|
|
|
Do both of the enantiomers work the same way?
|
No, each causes a different reaction
|
|
|
How do you define a optical isomer?
|
by the action of polarized light as it passes through a substance
EX: only the D form of the drug LSD is psychoactive Thalomide in its S form asts as a sedative, while in its R form it is associated with congenital abnormalities. |
|
|
Almost all anesthetic agents are?
|
organic compunds
|
|
|
Halogenation of hydrocarbons makes?
|
Inhalation gasses
|
|
|
What is used for a preservative of these gasses?
|
oxidized alcohols
|
|
|
What make up the local anesthetics?
|
amides and esters
|
|
|
Alkanes used for inhalation agents have what properties?
|
unstable and flammable
|
|
|
What does halogenation of these alkanes do?
|
Creates a highly potent, non flammable gas
hydrogen atom bond is replaced by a halogen atom, i.e. chlorine, fluorine, bromine. |
|
|
Enflurane and isoflurane are?
|
Isomers
Same chemical structure and weight, different physical characterisitics |
|
|
Halothane is?
|
halogenated hydrocarbon (only one that is), an ethane with fluorine, chlorine, and bromine
|
|
|
Is nitrous organic or inorganic?
|
Inorganic
|
|
|
If halothane is the only halogenated hydorcarbon, what are the rest?
|
Halogenated ethers, have an ether bridge (R-O-R) where R is an alkyl group
|
|
|
What is the IUPAC name for isoflurane
|
1-chlor-2,2,2-trifluoroethyl difluoromethyl ether
|
|
|
What is an ether?
|
R-O-R configuration, where the R is an alkyl group
Iso, des, and sevo are all methyl ethyl ethers |
|
|
Loacl anesthetics are made up of what?
|
Amides and esters
|
|
|
What is an amide? Examples?
|
when a hydrogen group is replaced with a amide group, CONH2
Ex: Lidocaine Bupivicaine |
|
|
What is an ester?
|
a molecule with a functional grou pof -COOR where R is the alkyl group
These are broken down by esterases |
|
|
What is the difference between natural and racemic epi?
|
Natural epi-exists in the L isomer and has greater beta 1 (cardiac) than beta 2 (pulmonary) activity
Racemic-kind used in the nebulizer equal amounts of l and d isomers get more pulmonary effects with less tachycardia. |
|
|
Since bupivicaine is cardiotoxic, what can be used to get he local effect without the cardiac side effects?
|
Robupivicaine, which is an isomer of bupivicaine
|
|
|
What is the structure of isoflurane?
|
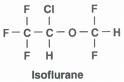
|
|
|
What is the structure of halothane?
|
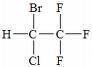
|
|
|
What is the structure of Desflurane?
|
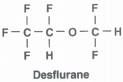
|
|
|
What is the structure of sevoflurane?
|
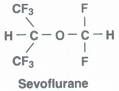
|
|
|
What is the chemical structure of enflurane?
|
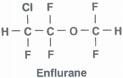
|
|
|
How is the acid base balance defined?
|
by the measurement of H ions
|
|
|
What regulates the balance between intake (production) and net removal of H ions form the body?
|
the body
|
|
|
How is the acid base balance measured?
|
pH scale
|
|
|
What is the pH scale?
|
power of hydrogen
a measure of the activity of hydrogen ions (H+) in a solution its acidity or alkalinity |
|
|
Ia a solution more basic or acidic when H+ ions = OH-?
|
neither, it is neutral.
|
|
|
What is the pH of water? What are you actually measuring?
|
pH=7
Actually measuring H3O+ (hydronium) OH- (hydroxide) |
|
|
What are the two principles of water?
|
Water Eq principle
Addition exponenets of H+ and OH- ion concentration always equal the exponent -14 Both H+ and OH- ions are always present in any solution Excess H+ = acidic sol Excess OH- = basic sol |
|
|
What does pH measure?
|
H+ ions in a sol
|
|
|
What produces H+ ions?
|
Acids
Acid pH < 7, a rough measure Reverse logarithmic representation of H+ concentration |
|
|
Explain how pH is figured.
|
pH 7 = H+ ion concentration of 0.0000001
H2O = 0.0000001, or pH 7 HCl has a pH of 2, so 0.01 if the ion concentration is 0.001, then pH = 3 The closer to one the ion concentration is, the more acidic it is. |
|
|
pH
|
units of moles of H+ per liter of sol
1 x 10(zero power) = pH 0 1 x 10(-4 power) = pH4, soda 1 x 10(-9 power) = pH9, NaHCO3 |
|
|
What is an acid?
|
any substance that when dissolved in water gives a sol with a pH of < 7.
|
|
|
What are two characteristics of an acid?
|
water soluble
sour tasting |
|
|
What are some reactions of an acid?
|
able to give up a proton (H+ ion) to a base
accept an unshared pare of electrons from a base reacts with a base in a neutralization reaction to form a salt acids increase the hydronium ion conc in water. |
|
|
What is a salt?
|
an ionic compound that is composed of positively charged cations and negatively charged anions
Neutral charge, no net charged Ionic bond |
|
|
Salt is a what? Example?
|
a base and an acid
Ammonia and hydrochloric acid = ammonium chloride NH3 + HCL = NH4CL |
|
|
What is meant by alkaline?
|
A chemical compound that absorbs hydronium ions when dissolved in water (proton acceptor)
bases reduce the concentration of hydronium ions (HCO3+) in water |
|
|
What are some characteristics of bases?
|
less viscous than pure water
bitter taste soapy to the touch |
|
|
Acids and bases act how in water?
|
An acid in water gives up a H to the water; HCL in H2O = H+ and CL-
A base in water gives up OH to the water: NaOH in H2O = Na+ and OH- |
|
|
What is neutralization?
|
mix an acid/base together
H+ + OH- = H2O H20 has a pH7 How a salt is formed Salt = no charge |
|
|
What is hydrolysis?
|
Formation of water and a salt from an acid and base.
EX: HCl + NaOH = H2O + NaCl (salt) HBr + KOH = H2O + KBr (salt) |
|
|
An acid and base can also be combine to give a weaker acid and a salt.
|
HCL + NaHCO3 = H2CO3 + NaCl
Hydrochloric acid and sodium bicarb = carbonic acid and sodium chloride |
|
|
What is a conjugate?
|
Each acid has a conjugate base
Each base has a conjugate acid Conjugate pairs only differ by a proton (either have one more or less H) acids always have one more H, bases one less The conjugate base of a weak acid is a strong base, and the conjugate base of a strong acid is a weak base. |
|
|
What are some examples of conjugates?
|
H2SO4 Sulfuric acid
HSo4- Hydrogen sulfate(base) H3O+ Hydronium ion (acid) H2O Base NH4+ Ammonium ion (acid) NH3 Ammonia (base) |
|
|
How do the acid base balances play out when mixed together in regards to pH?
|
Acid Base Salt pH
--------------------------------------------------- Strong Strong pH=7 Strong Weak pH<7 Weak Strong pH>7 Weak Weak Stronger one |
|
|
Buffers are what?
|
solutions that resist change in pH upon addition of small amounts of acid or base
|
|
|
How are buffers represented?
|
salt/acid or conjugate base/acid
|
|
|
What are buffers useful for?
|
chemical manufacturing
many biochemical processes present in blood plasma carbonic acid (H2CO3) bicarbonate (HCO3-) |
|
|
What are some important values for pH in regards to human life?
|
pH 7.40 neutral
pH < 7.0 is life threatening pH > 7.8 is morbidity |
|
|
What is the key to pH regulating?
|
hydrogen ion concentration
|
|
|
How are hydrogen ions regulated?
|
buffer systems
|
|
|
What is the ECF/ICF primarily buffered by?
|
ECF= HCO3 (bicarb) and CO2
ICF= proteins and PO4 |
|
|
What are two important acids? Roles?
|
Carbonic acid
H2CO3 Metabolism of carbohydrates and fats; 15,000 mmol of CO2/day Non carbonic acids Protien metabolism; 1.5 mmol H+ ions/kg/day. |
|
|
How are buffers classified?
|
Chemical
In cells and body fluids, react immediately (fastest) Respiratory CO2, react within minutes (fast) Metabolic HCO3-, react within hours (slowest) |
|
|
What are the main three buffer systems in the body?
|
Blood (fastest)
Respiration (CO2) Kidneys (HCO3-) Acid base homeostasis centers around regulation of these |
|
|
What are three key features of the HCO3-/CO2 (Carbonic acid-Bicarbonate) buffer system?
|
it is the buffer present at the highest concentrations in the body
its pKa value of 6.1 is close enough to the physiologic pH to make it a good buffer the major components of the buffer system can be independently regulated by the lungs (CO2) and kidneys (HCO3-) |
|
|
How does dietary intake affect acid/base?
|
normal caloric intake of a non meat based diet produces 20,000 meq of acid/day. CO2 is produced as the end product of carb and fat metabolism.
The CO2 is excreted by the lungs |
|
|
Protein catabolism contributes how?
|
1meq/kg (50-60meq-day) of inorganic acids produced
controlled by kidneys through excretion and HCO3- formation |
|
|
What is the bicarbonate buffer system?
|
H + HCO3 = H2CO3 = CO2 + H2O
(this is a reversible equation) increased H+ will bind with HCO3- Need carbonic anhydrase to function properly then forms carbonic acid H2CO3 Carbonic acid immediately breaks down into CO2 and H2O Inc H+ leads to inc CO2 and inc resp and depth Dec H+ leads to dec CO2 and dec resp and depth (Resp sys compensates by changing resp depth/rate) |
|
|
What is the slowest of the buffer systems?
|
Renal
|
|
|
How does the renal buffer system work?
|
Dec pH leads to excretion of H+ ions in urine
Inc pH leads to reabsorbtion of H+ ions back into the urine takes hours or even days for compensation |
|
|
What does the hemoglobin buffer system do?
|
Prevents drastic changes in the plasma CO2
RBC absorbs CO2, changes it to H2CO3 (carbonic acid) which then quickly disassociates |
|
|
How does the Hgb buffer system work?
|
CO2 + H20 -> HbCO2 or HbO2 -> H+
+ HCO3- HbCO2 carried to the lungs-CO2 blown off HbO2 dumps the O2 into tissues H+ ions bufferd by Hgb (HHb) Chloride shifts into RBC's, and HCO3- shifts out. |
|
|
What does the respiratory buffer system do?
|
works with the carbonic acid/bicarb system
goes from muscle to blood plasma to lungs CO2 + H2O -> H and HCO3 -> CO2 and H2O |
|
|
Where is the phosphate buffer system found?
|
ICF and urine
Na2HPO4 <-> 2Na + HPO4 + H |
|
|
How does the body maintain pH?
|
Blood
Carotid and aortic sinuses CSF (slower to change pH, takes longer to change. For ex, if correct pH in blood, nay not show in CSF for while) Receptors in medulla oblongata sense pH probs Changes in rate/depth of resps maintain pH |
|
|
Hoe does the renal sys compensate for acid base imbalances?
|
H+ is secreted into renal tubules and stops at urine pH of approx 4.5; other buffers keep the pH high enough so the secretion can continue
Changes rate of secretion/absorbtion of H+ and HCO3- |
|
|
What is the biggest problem with acid base balance?
|
elimination of excess acid
|
|
|
Where do acids come from?
|
Normal metabolism
Ingested acids |
|
|
What are the two forms of acids?
|
Volatile = H2CO3
Non volatile = products of metabolism |
|
|
What is a volatile acid?
How is it eliminated? |
H2CO3, carbonic acid
Weak acid, doesn't release H+ easily Needs carbonic anhydrase for dissociation Eliminated by lungs |
|
|
What is a volatile acid generated from?
|
Normal metabolism of
Fat Carbs Some proteins |
|
|
What is a non volatile acid?
How is it eliminated? |
H2SO4, H3PO4 form metabolism of:
Sulfur containing products Phosphoproteins Eliminated by kidneys |
|
|
What are some products of intermediary metabolism of non volatile acids?
|
Lactic acids/ ketoacids
|
|
|
When excess acid or or base is introduced into the body, what happens?
|
it is immediately bufferred to minimize changes in the pH
|
|
|
A good buffer should maintain a pH of what?
|
7.4
|
|
|
What is pKa?
|
A measure of the tendency of a molecule or ion to keep a proton
H+ Acids give up/lose H+ |
|
|
Acid Dissociation constant is...
|
the acid ionization constant or Ka
indicates the dissociation of hydrogen ions from an acid strong acids dissociate practica;ly completely in solution |
|
|
pKa with strong/weak acids
|
Strong
large acidity constants Close to 1 Weak do not fully dissociate acidity constants far less than one |
|
|
Review of acids/pH
|
stronger acids are closer to 1
HCL pH2 = 0.01 (close to 1) weak acids are farther from 1 Soda pH4 =0.0001 |
|
|
All drugs exist in ____ forms? What are they?
|
2
Ionized and non ionized |
|
|
When the pH is 7.4 and the pKa is 7.4, what is the ratio of ionized to non?
|
50/50
|
|
|
If the pKa of a drug is 8.1 and the pH is 7.4, is the ratio mentioned above still true?
|
No, there is no longer a 50/50 mix non to ionized drug.
|
|
|
Which buffers are the best?
|
Buffers with a pKa closest to 7.4 are the best
Need to be present in large amounts |
|
|
Do H+ ions move freely between the ECF and ICF?
|
Yes
|
|
|
How is the movement of H+ into/out of the cell handled?
|
H+ movement must be matched
Negatively charged ion (anion) moving in same direction or positively charged (cation) in the opp direction. So, + and - out or + in and + out. Inc influx of H+ into the cell elicits an inc move of K+ out of cells into ECF. H+ in, K+ out |
|
|
What are the most abundant buffers in and out of the cells? Why?
|
Proteins, because have the aa histidine (contains a imidazole ring with pKa of 7.0)
|
|
|
Which protein is the best buffer?
|
Hemoglobin, it contains an unusually large number of histidines; 36 per molecule
|
|
|
Where are phospate buffers located?
|
present in large amounts inside of the cells
small amounts in the ECF the pKa of HPO42+/H2PO4- is 6.8, makes a good intracellular buffer |
|
|
With the bicarb/CO2 buffer system, what happens to the acid and base?
|
H+ + HCO3- <--> H2CO3 <--> CO2 + H2O (facilitated by carbonic anhydrase located in the RBC)
The CO2 (acid) is exhaled Acid is gone, but the base was lost in the transformation |
|
|
How is the bicarb regenerated?
|
Regenerate by bone stores of carbonate (not enough to keep up with loss)
so.. Kidney is able to regenerate new bicarb to replace that which was lost |
|
|
How is the bicarb regen (cont)?
|
Kidneys work like this:
CO2 + H2O <--> H2CO3 <--> H+ HCO3- H+ is secreted into renal tubule Buffered by: HPO4 = Phosphate NH3 = Ammonia H+ secreted into urine HCO3- reabsorbed into plasma |
|
|
Does the Bicarb/CO2 buffer system run in just one direction?
|
no, goes in both directions
respiration's increase/decrease with changes to pH renal function also changes. |
|
|
The basic action of an anesthetic in the body is largely a function of
|
its chemical structure and the resulting interaction with a cellular receptor complex
|
|
|
What plays a major role in modern anesthesia?
|
Gasses
|
|
|
Are gasses the only anesthetic used?
|
No, unlike days of old where only one gas may had been used, now many gasses and IV drugs are used
|
|
|
How is anesthesia administered?
|
1. Liquid --> Vaporize it
2. Controlled vapor delivery to lungs --->bloodstream --->BRAIN-spinal cord ---> other places i.e. fat tissue |
|
|
What is the major assumption regarding lungs and brain with partial pressure?
|
The partial pressure in the lungs is equivalent to that present in the brain because they are highly lipid soluble and diffusible (I.e. equilibrium is quickly achieved.
|
|
|
What is MAC?
|
Minimum alveolar concentration is the concentration necessary to produce lack of movement to 50% upon surgical stimulation
(Due to the high lipid solubility, pressure in the lungs is assumed to be the same as in the brain). |
|
|
How fast is equilibrium achieved between body compartments?
|
quickly, and the faster the lung (brain) concentration rises the faster anesthesia is achieved; the opposite is also true, the faster the concentration falls the quicker the patient emerges.
|
|
|
What are some factors that influence the ability of a gas to anesthetize patient?
|
Blood/gas solubility
Oil/gas sol Ventilation Concentration Circulation Metabolism |
|
|
What is the blood/gas solubility?
|
A coefficient that indicates the speed of uptake and elimination
Reflects the portion of anesthetic that will bind to blood components versus that which will leave the blood for the tissues (2.3:1) The more sol the drug (high blood gas coef) the slower the brain uptake |
|
|
What is oil/gas sol?
|
Indicator of potency
the higher the oil/gas sol, the more potent the drug at the tissue level, the lipid sol allows it to penetrate membranes and produce action |
|
|
Principles of ventilation
|
The diffusible drug moves down a concentration gradient
The faster and deeper the patient breathes the faster they are anesthetized (true for emergence as well) A VQ mismatch or poor lung function hinders administration The amount of gas in the alveoli is r/t the anesthetic depth |
|
|
Principles of concentration
|
higher conc speeds uptake
over pressuring can speed uptake of more soluble (slower) agents, but is less effective with the faster agents admin of a slow agent with a faster agent at the same time can speed the uptake of the slower agent (2nd gas effect) |
|
|
What two gasses are used for the 2nd gas effect?
|
Halothane and N2O
Halo Oil/gas coef 224 (Very potent) Blood gas coef 2.3 (very blood sol, not much to tissues, takes longer) N2O Oil/gas coef 1.4, so not very potent Blood gas sol is .47, so not ver blood sol |
|
|
WHat is diffusion hypoxia?
|
when one turns off the flow of Nitrous Oxide at the end of anaesthesia, then the concentration in the alveoli is lower than in the blood. Consequently N20 floods in from the blood, usurping the 02 and N2 in the process
|
|
|
How can diffusion hypoxia be avoided?
|
admin of 100% O2for 3-5 min upon discontinuation of nitrous
|
|
|
Principles of circulation
What is the importance of circulation? |
without it, the drug would never reach the lungs and brain
|
|
|
Is increased cardiac output good or bad when anesthetizing a patient?
|
Inc cardiac output is detrimental, as inc output leads to greater lung perfusion and more of the drug absorbed into the blood, thus slowing rise in the brain concentration.
|
|
|
As the length of time an anesthetic is admin, what happens?
|
more anesthetic reaches the vessel poor groups
|
|
|
Are gases metabolized in the body?
|
yes, to varying degrees. MOst of the drug is simply blown off
|
|
|
What are halothane and sevo associated in regards to toxicity?
|
Halo ---> Hepatotoxicity
Sevo ---> nephrotoxicity |
|
|
What is a Carbon dioxide scrubber? Where are they used?
|
A mixture of chemicals used in granular form in a closed breathing environment
they are used in anesthesia and in submarines |
|
|
What does a CO2 scrubber do?
|
Removes CO2 from breathing gasses to prevent CO2 retention and carbon dioxide posioning
|
|
|
What types of Scrubbers exist?
|
Soda Lime
Baralyme Amsorb Medisorb Dragersorb |
|
|
What is the common link between all of the scrubbers?
|
All contain water
|
|
|
What is the importance of water in the scrubber?
|
water is needed for the chemical reactions to take place
|
|
|
What are some characteristics of the scrubbers?
|
Mesh 4-8
With these systems, there is a compromise between absorbative capacity and resistance to airflow. Smaller granules allow more surface area, however they pack tighter and increase resistance to flow. |
|
|
How does one know when the granules are bad/scrubber needs replaced?
|
Ethyl violet in the granules changes color to purple/blue indicating need to change
|
|
|
If water is dried out or missing, can the scrubber still be used?
|
NO, water is necessary for the chemical reactions. If water is missing, the scrubber gets hotter and hotter-may cause a fire
|
|
|
What is the most common absorbent used?
|
soda lime
|
|
|
Does soda lime produce an exo or endothermic reaction?
|
exothermic
|
|
|
What chemicals are contained in the soda lime?
|
Calcium hydroxide Ca(OH)2 75%
Water (H2O) 20% Sodium hydroxide (NaOH) 3% Potassium hydroxide (KOH) 1% Caustic agents |
|
|
Sevo is unstable in soda lime. What does it produce?
|
Compound A
|
|
|
What are some characteristics of compound A?
|
lethal at 130-340ppm
Renal injury has been documented in rats at 25-50ppm (never in humans) Avoid gas flows of 1L/min for more than 2 MAC hours |
|
|
What is the activator in soda lime?
|
NaOH or KOH
|
|
|
Do carbon monoxide problems occur with ethyl methyl ethers?
|
Yes
|
|
|
What is the activator in baralyme?
|
Ba(OH)2 Barium hydroxide
|
|
|
Is baralyme more or less efficient than soda lime?
|
less efficient (questionable)
|
|
|
Where is baralyme most often used? Why?
|
Dry climates, water is in small particles and baralyme is less likely to dry out. (retains water well, desert storm)
|
|
|
What is the activator for Amsorb?
|
Ca(OH)2 Calcium hydoxide
|
|
|
What is amsorb suitable for?
|
low flow anesthesia.
|
|
|
The presence of sodium hydroxide at any level provides the basis for what?
|
Anesthetic agent dehalogenation
|
|
|
What are some complications of dessicated (dehydrated) CO2 absorbents?
|
Exothermic reactions
Production of: Compound A Carbon Monoxide Methanol Formaldehyde |
|
|
What are exothermic reactions associated with?
|
Sevo, enhanced by the type of stong base used
KOH>NaOH Can also happen with other gasses, however the greatest incidence is with sevo and baralyme (KOH) |
|
|
CO production is greatest with ...
|
dessicated KOH
|
|
|
Is the CO produced usually detected by routine monitors?
|
No
|
|
|
CO production correlates with...
|
In descending order:
Des Enflurane Iso CHF2 group |
|
|
Compound A is a potential...
|
nephrotoxin
Produced by Sevo and dry or wet CO2 absorbent |
|
|
Can soda lime change back to its original color if it sits long enough?
|
yes
|
|
|
Does soda lime change color when it is dried up?
|
No
|
|
|
When the container is dried up, what can happen?
|
CO production and exothermic reactions
|
|
|
When the container is used up, what can happen?
|
no breakdown of CO2
|
|
|
How can complications be prevented with scrubbers?
|
Turn O2 off at the end of each case
Turn machines off at the end of each day Place date changed on canister Question temp stickers on canisters |
|
|
What is the soda lime conversion formula?
|
H2O + CO2 = H2CO3 = H+ + HCO3-
then NaOH +H2CO3 = NaHCO3 +H20 then 2NaHCO3 + Ca(OH)2 = 2NaOH + CaCO3 + H20 |
|
|
What is the baralyme converson formula?
|
Ba(OH)2-8 H2O + CO2 --> BaCO3 + 9 H2O + energy
then 9 H2O + 9 CO2 ---> 9H2CO3 then 9 H2CO3 + 9Ca(OH)2 --->CaCO3 + 18H20 + energy CaCO3 is an insoluble precipitate |
|
|
What are the tank sizes? Amounts?
|
E cylinder
2 feet long, 4 inches diam Filled to 2200 psi @ ROOM TEMP 660L H cylinder 4 feet long, 9 inches long Filled to 2200 psi @ ROOM TEMP 6900L |
|
|
Why/How is N2O different?
|
Needs to be changed @ approx 750psi
Tank holds 1590L of nitous oxide at one atmosphere pressure |
|
|
Can the amount remaining in the N2O cylinder be determined by pressure gauge?
|
No, must be weighed
Subtract the tare weight from weight of empty tank to get remaining |
|
|
What are the tank colors?
|
O2 green
CO2 gray N20 blue Cyclopropane orange Helium brown Nitrogen black Medical air yellow |
|
|
After pipelines are installed in an institution, pressure regulators are installed that function to maintain normal outlet pressure. What is that pressure?
|
55 psi (O2)
|
|
|
Are O2 tanks ever allowed to be free standing?
|
no
|
|
|
What are some cylinder safety characteristics?
|
walls 3/8" thick
Cylinders tested at 1.66 service pressure Pressure tested every 5 years |
|
|
What is the critical temp of O2?
N2O? |
-119C
39.5C |
|
|
What is critical temp?
|
Gasses liquify if sufficient pressure is applied and the temp is below the critical value called the critical temp.
A gas can not be liquified if the temp is above the critical temp. So... O2 CT is -119C, so will always be a gas at temps -118 or greater N2O CT is 39.5, so at room temp (20C), it will be liquid. Below CT = LIquid Above CT = Gas |
|
|
How does N2O get form a liquid to a gas for patient admin?
|
N2O vapor is vaporized and in EQ over the liquid state
|
|
|
Who sets the standards and guidelines for tank safety?
|
OSHA (health and safety of workers)
FDA (standards for med devices and gasses-still a drug) Pharmacopeia of the US and National formulary (deelops purity specs) |
|
|
Pressure relief valves-are they required?
|
Yes, on aLL MED GAS CYLINDERS
|
|
|
What are the three types of pressure relief valves?
|
Fusible plug
Frangible disc assembly Safety release valve |
|
|
What is the fusible plug?
|
Woods metal, metal alloy that has a low melting point
|

