![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
294 Cards in this Set
- Front
- Back
|
Oncogene pg. 343-346, 369
|
is a gene that, when mutated or expressed at high levels, helps turn a normal cell into a tumor cell.[1]
|
|
|
Proto-oncogene p343
|
is a normal gene that can become an oncogene due to mutations or increased expression. Proto-oncogenes code for proteins that help to regulate cell growth and differentiation. Proto-oncogenes are often involved in signal transduction and execution of mitogenic signals, usually through their protein products. Upon activation, a proto-oncogene (or its product) becomes a tumor-inducing agent, an oncogene.[7] Examples of proto-oncogenes include RAS, WNT, MYC, ERK, and TRK.
|
|
|
Carcinoma p333-409
|
carcinoma is any malignant cancer that arises from epithelial cells. Carcinomas invade surrounding tissues and organs and may metastasize, or spread, to lymph nodes and other sites.
|
|
|
Sarcoma p335-336
|
(from the Greek 'sarx' meaning "flesh") is a cancer of the connective tissue[1] (bone, cartilage, fat) resulting in mesoderm proliferation. This is in contrast to carcinomas, which are of epithelial origin (breast, colon, pancreas, and others). However, due to an evolving understanding of tissue origin, the term "sarcoma" is sometimes applied to tumors now known to arise from epithelial tissue.[3] The term soft tissue sarcoma is used to describe tumors of soft tissue,[4] which includes elements that are in connective tissue, but not derived from it (such as muscles and blood vessels).
|
|
|
Benign p333
|
implies a mild and nonprogressive disease, and indeed, many kinds of benign tumors are harmless to the health. However, some neoplasms which are defined as 'benign tumors' because they lack the invasive properties of a cancer, may still produce negative health effects. Examples of this include tumors which produce a "mass effect" (compression of vital organs such as blood vessels), or "functional" tumors of endocrine tissues, which may overproduce certain hormones (examples include thyroid adenomas, adrenocortical adenomas, and pituitary adenomas).
Benign tumors typically are encapsulated, which inhibits their ability to behave in a malignant manner. Nonetheless, many types of benign tumors have the potential to become malignant and some types, such as teratoma, are notorious for this. |
|
|
Malignant p334-336
|
from the Latin roots mal- = "bad" and -genus = "born") is the tendency of a medical condition, especially tumors to become progressively worse and to potentially result in death. It is characterized by the properties of anaplasia, invasiveness, and metastasis.[1] Malignant is a corresponding adjectival medical term used to describe a severe and progressively worsening disease. The term is most familiar as a description of cancer. A malignant tumor may be contrasted with a non-cancerous benign tumor in that a malignancy is not self-limited in its growth, is capable of invading into adjacent tissues, and may be capable of spreading to distant tissues (metastasizing), while a benign tumor has none of those properties. Malignant tumor is synonymous with cancer.
|
|
|
Metastasis or metastatic disease, sometimes abbreviated mets p375, 381-384
|
is the spread of a disease from one organ or part to another non-adjacent organ or part. Only malignant tumor cells and infections have the established capacity to metastasize; however, this is recently reconsidered by new research, Cancer cells can break away, leak, or spill from a primary tumor, enter lymphatic and blood vessels, circulate through the bloodstream, and be deposited within normal tissue elsewhere in the body. Metastasis is one of three hallmarks of malignancy (contrast benign tumors).[4] Most tumors and other neoplasms can metastasize, although in varying degrees (e.g., glioma and basal cell carcinoma rarely metastasize).[4]
When tumor cells metastasize, the new tumor is called a secondary or metastatic tumor, and its cells are like those in the original tumor. This means, for example, that, if breast cancer metastasizes to the lungs, the secondary tumor is made up of abnormal breast cells, not of abnormal lung cells. The tumor in the lung is then called metastatic breast cancer, not lung cancer. |
|
|
Neoplasm p333
|
- is an abnormal mass of tissue as a result of neoplasia. Neoplasia (new growth in Greek) is the abnormal proliferation of cells. The growth of this clone of cells exceeds, and is uncoordinated with, that of the normal tissues around it. It usually causes a lump or tumor. Neoplasms may be benign, pre-malignant or malignant.
In modern medicine, the term tumor is synonymous with a neoplasm that has formed a lump. In the past, the term tumor was used differently. Some neoplasms do not cause a lump. |
|
|
Tumor Suppressor Gene –or antioncogene p343-346, 369
|
is a gene that protects a cell from one step on the path to cancer. When this gene is mutated to cause a loss or reduction in its function, the cell can progress to cancer, usually in combination with other genetic changes.
|
|
|
Transformation p337, 375
|
is the genetic alteration of a cell resulting from the uptake, genomic incorporation, and expression of foreign genetic material (DNA).[1]
Separate terms are used for genetic alterations resulting from introduction of DNA by viruses ("transduction") or by cell-cell contact between bacteria ("conjugation"). Transformation of eukaryotic cells in tissue culture is usually called transfection. RNA may also be transferred into cells using similar methods, but this does not normally produce heritable change and so is not true transformation. |
|
|
Undifferentiated p1239-1240, 1239
|
is the process by which a less specialized cell becomes a more specialized cell type. Differentiation occurs numerous times during the development of a multicellular organism as the organism changes from a single zygote to a complex system of tissues and cell types. Differentiation is a common process in adults as well: adult stem cells divide and create fully-differentiated daughter cells during tissue repair and during normal cell turnover. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly-controlled modifications in gene expression. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. Thus, different cells can have very different physical characteristics despite having the same genome.
|
|
|
Carcinoma in situ p336-337
|
Carcinoma, like all neoplasia, is classified by its histopathological appearance. Adenocarcinoma and squamous cell carcinoma, two common descriptive terms for tumors, reflect the fact that these cells may have glandular or squamous cell appearances respectively. Severely anaplastic tumors might be so undifferentiated that they do not have a distinct histological appearance (undifferentiated carcinoma).
Sometimes a tumor is referred to by the presumptive organ of the primary (eg carcinoma of the prostate) or the putative cell of origin (hepatocellular carcinoma, renal cell carcinoma). Metastatic carcinoma can be diagnosed through biopsy, including fine-needle aspiration, core biopsy, or subtotal removal of single node. |
|
|
Describe the two mutational routes that result in uncontrolled cellular proliferation.
|
The two mutational routes would be anaplasia, or loss of differentiation, and autonomy, or independence from normal cellular controls
|
|
|
Describe how autonomy and anaplasia define cancer.
|
In the adult, undifferentiated cells (not totally committed to a specific function) are known as pluripotent cells, precursor cells or stem cells. Cancer cells become more like embryonic cells and are less differentiated. Cancerous growth depends on derangements of cell differentiation.
|
|
|
State how tumors are graded p384
|
The staging of cancers is the extent of spread of the neoplasm. Grading is the system used to record the tumors degree of differentiation from the parent tissue. High grade lesions shows little differentiation and may convey a worse prognosis depending on tumor type. As a general rule, cancer stage (using AJCC criteria) dictates ultimate prognosis. The criteria for staging differ based on organ system. For example, the colon and bladder cancer staging system relies on depth of invasion. Breast and lung staging is more dependent on size. While renal carcinoma staging is based on both size and invasion into the renal sinus. Accurate staging is reliant on clinical, radiographic, and pathologic data. The UICC/AJCC TNM system is often used, however for some common tumors, classic staging methods (such as the Dukes classification for colon cancer) are still used.
|
|
|
Discuss the changes cell surfaces go though and their functional importance in cancer.
|
It takes 5-6 distinct mutations in different signaling pathways to produce cancer. Maturations activate growth-promotion pathways, block antigrowth signals, prevent apoptosis, turn on telomerase and new blood vessel growth, and allow tissue invasion and distant metastasis.
|
|
|
Explain the role of tumor cell markers in diagnosis and treatment of specific types of cancer.
|
Tumor markers are substances (i.e. hormones, enzymes, genes, antigens, antibodies) found on tumor plasma membranes and in the blood, spinal fluid, or urine. They are used to screen and identify individuals at high risk for cancer to help diagnose specific types of tumors. And to follow the clinical course of cancer.
|
|
|
Discuss the initiation-promotion-progression theory of carcinogenesis p354-355
|
Three main genetic mechanisms have a role in human carcinogenesis (a) mutation of proto-oncogenes resulting in hyperactivity of growth-related gene products (such genes are called oncogenes); (b) mutation of genes resulting in loss or inactivity of gene products that normally would inhibit growth (such genes are called tumor suppressor genes) ; and (c) mutation of genes resulting in over expression of products that prevent normal cell death, or apoptosis, thus allowing continued growth of tumors.
|
|
|
|
|
|
|
phase 1 of immunoediting
|
The first phase of elimination involves the initiation of antitumor immune response. Cells of the innate immune system recognise the presence of a growing tumor which has undergone stromal remodeling, causing local tissue damage. This is followed by the induction of inflammatory signals which is essential for recruiting cells of the innate immune system (eg. natural killer cells, natural killer T cells, macrophages and dendritic cells) to the tumor site. During this phase, the infiltrating lymphocytes such as the natural killer cells and natural killer T cells are stimulated to produce IFN-gamma.
|
|
|
phase 2 immunoediting
|
The first phase of elimination involves the initiation of antitumor immune response. Cells of the innate immune system recognise the presence of a growing tumor which has undergone stromal remodeling, causing local tissue damage. This is followed by the induction of inflammatory signals which is essential for recruiting cells of the innate immune system (eg. natural killer cells, natural killer T cells, macrophages and dendritic cells) to the tumor site. During this phase, the infiltrating lymphocytes such as the natural killer cells and natural killer T cells are stimulated to produce IFN-gamma.
|
|
|
phase 3 immunoediting
|
In the third phase, natural killer cells and macrophages transactivate one another via the reciprocal production of IFN-gamma and IL-12. This again promotes more tumor killing by these cells via apoptosis and the production of reactive oxygen and nitrogen intermediates. In the draining lymph nodes, tumor-specific dendritic cells trigger the differentiation of Th1 cells which in turn facilitates the development of CD8+ T cells.
|
|
|
phase 4 immunoediting
|
In the final phase of elimination, tumor-specific CD4+ and CD8+ T cells home to the tumor site and the cytolytic T lymphocytes then destroy the antigen-bearing tumor cells which remain at the site.
|
|
|
equilibrium and escape of immunoediting
|
Tumor cell variants which have survived the elimination phase enter the equilibrium phase. In this phase, lymphocytes and IFN-gamma exert a selection pressure on tumor cells which are genetically unstable and rapidly mutating. Tumor cell variants which have acquired resistance to elimination then enter the escape phase. In this phase, tumor cells continue to grow and expand in an uncontrolled manner and may eventually lead to malignancies. In the study of cancer immunoeditting, knockout mice have been used for experimentation since human testing is not possible[2]. Tumor infiltration by lymphocytes is seen as a reflection of a tumor-related immune response[7].
|
|
|
Genetic mutation severity
|
The frequency and consequences of genetic mutations can be altered by a number of environmental factors. The most significant factors include smoking, radiation, obesity, and a few oncogenic viruses.
|
|
|
Smoking and cancer p353
|
Since 1950’s death attributable to smoking increased. Smoking causes cancer of the renal gland, bladder, penis, Oral cavity, pharynx, larynx, nasal cavities, sinuses, esophagus, stomach, liver, kidney , uterus, and myeloid leukemia. Involuntary smoking also causes ca of the lungs.
|
|
|
Mechanisms of carcinogenesis from smoking p353-355
|
Polycylic aromatic hydrocarbons and mutations in p53, nitroso compounds, arylamines form DNA adducts in bladder cells, and benzopyrene metabolites found in cervical mucus is related to DNA adducts, however, studies did not adjust for HPV and smokers have higher levels of benzene an inducer of leukemia.
|
|
|
Health risks of ionizing radiation
|
Involve neoplastic diseases but also birth defects and risk of low dose radiation is being debated.
|
|
|
Severity of radiation induced damage
|
Depends on dose response , LET, fractionation, protraction, repair mechanism, bystander effects, and antioxidants.
|
|
|
Dose response models
|
Used to estimate cancer risk from radiation
1) Linear- no-threshold, relationship because any dose has the potential to cause cancer 2) A threshold dose below that which radiation may not cause cancer currently the validity of this theory is questionable. |
|
|
Progeny and irradiated cells
|
Can exhibit an increased death rate and loss of reproductive potential
|
|
|
Bystander effects and genomic instability p354-355
|
Low levels of radiation can induce this. Both finding appear to be associated with oxidative stress and cell-to-cell intercellular communication.
|
|
|
Reactive oxygen species (ROS) (oxidative stress) p52-53
|
Involved in skin carcinogenesis from ultraviolet light (UV)
|
|
|
B-raf proto-oncogene
|
Noted in 60-70% of melanoma cell lines and tissues. UVB light and sunburn triggers inflammation causing cytokine release and activation of growth factors that may be related to this mutation.
|
|
|
Chronic alcoholism
|
Strong factor in cancer of the oral cavity, pharynx, hypopharynx, larynx, esophagus, and liver. It is less strongly related to breast ca, and colorectal cancer although breast carcinogens can be enhanced with relatively low daily amounts
|
|
|
Mechanism of ETOH carcinogenesis
|
Multiple mechanisms are involved in and include acetaldehyde, induction of cytochrome p-450 and ROS, increased procarcinogen activation, cell cycle effects, and nutritional deficiencies.
|
|
|
Development of cervical cancer p795-797
|
Sexually transmitted infection with high risk types of HPV is required for the development.
|
|
|
Effect of physical activity on breast cancer
|
Physical activity reduces the risk
|
|
|
Effect of occupational factors on cancer
|
A substantial percentage of cancers of the upper respiratory tract, lung, bladder, and peritoneum are attributed to occupational factors.
|
|
|
Air pollution and cancer
|
This is a concern in regard to cancer because of the inhalation of emissions, including arsenicals, benzene, chloroform, vinyl chloride, and acrylonitrile. Inside pollution is a greater concern because of cigarette smoke and possibly radon gas.
|
|
|
Electromagnetic fields (EMF’s) and cancer
|
This link is controversial The evidence does not provide clear or consistent results, however, the results cannot establish the absence of any hazard.
|
|
|
Obesity and cancer
|
High BMI is associated with higher rates of death from esophageal, stomach, colorectal, liver, breast, gallbladder, pancreas, prostate, kidney, non-Hodgekin, ovarian, lymphoma, multiple myeloma, cervical, and leukemia
|
|
|
Adipose tissue and cancer
|
This is an active endocrine and metabolic tissue. Increased release of free fatty acids, resistin, TNF-α, and reduced release of adiponectin give rise to insulin resistance. These cells produce steroid-hormone-metabolizing enzymes and are an important source of estrogens in postmenopausal women. Insulin-like growth factor-1 (IGF-1) regulates cell proliferation and inhibits apoptosis and the synthesis and biologic availability of female and male sex hormones.
|
|
|
Historic analysis of theories of cancer invasion and metastasis p375
|
Began with the black bile diffusion theory of Hippocrates and later included such theories as the cellular-embolic theory and the seed-versus soil theory.
|
|
|
Current theories of cancer invasion and mets
|
Emphasis is on the biochemical mechanisms of metastasis, including control of proliferation, cell-cell and cell-matrix interaction, angiogenesis, migration, invasion, and dissemination.
|
|
|
Describe the theory of invasion as stated in the text
|
This is a prerequisite for metastasis and first step to the metastatic process. The progression of a tumor cell from being one of benign proliferation to one that invade and develops metastatic growth is the major cause of poor clinical outcome of individuals with cancer. In its earliest stages this may occur as a function of direct tumor extension. Eventually cells or clumps of cells become detached or lose cell-to-cell adhesion to the primary tumor and invade the surrounding interstitial spaces.
|
|
|
diapedesis p191
|
The blood vessels are lined with the endothelium, a layer of cells that tends to protect blood cell migration outside of the cells. However, injury or trauma can cause white blood cells to migrate across the endothelium. This process is called diapedesis.
As a result of diapedesis, white cells become part of the interstitial fluid, which surrounds the blood vessels and the tissue cells of the body. The white cells may exhibit diapedesis to fight infection in the tissues surrounding blood vessels. The blood vessels themselves provide a built-in pathway for diapedesis to occur when needed. |
|
|
Pluripotentail p902
|
Able to develop into or effect any (or most) cell type i.e. not restricted to a specific system
|
|
|
Hemes
|
A heme (American English) or haem (British English) is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are known as hemoproteins.
|
|
|
Erythropoesis p909
|
Erythropoiesis is the process by which red blood cells (erythrocytes) are produced and it is caused by iron released when a red blood cell dies.[1] In human adults, this usually occurs within the bone marrow. In the early fetus, erythropoiesis takes place in the mesodermal cells of the yolk sac. By the third or fourth month, erythropoiesis moves to the spleen and liver.[2] In humans with certain diseases and in some animals, erythropoiesis also occurs outside the bone marrow, within the spleen or liver. This is termed extramedullary erythropoiesis.
The tibia and femur cease to be important sites of hematopoiesis by about age 25; the vertebrae, sternum, pelvis and ribs, and cranium bones continue to produce red blood cells throughout life. |
|
|
Bands p896
|
A band cell (or band neutrophil) is a cell undergoing granulopoiesis, derived from a metamyelocyte, and leading to a mature granulocyte.
It is characterized by having a nucleus which is curved, but not lobar.[1] Often the term "band cell" implies a neutrophilic lineage. However, the term is not used only with neutrophils.[2] A count of band neutrophils is used to measure inflammation. An excess is called bandemia. |
|
|
Totipotential
|
Totipotency is the ability of a single cell to divide and produce all the differentiated cells in an organism, including extraembryonic tissues. [1] Totipotent cells formed during sexual and asexual reproduction include spores and zygotes. Zygotes are the products of the fusion of two gametes (fertilization). In some organisms, cells can dedifferentiate and regain totipotency. For example, a plant cutting or callus can be used to grow an entire plant.
Human development begins when a sperm fertilizes an egg and creates a single totipotent cell (zygote). In the first hours after fertilization, this cell divides into identical totipotent cells. Approximately four days after fertilization and after several cycles of cell division, these totipotent cells begin to specialize. Totipotent cells have total potential. They can specialize into pluripotent cells that can give rise to most, but not all, of the tissues necessary for fetal development. Pluripotent cells undergo further specialization into multipotent cells that are committed to give rise to cells that have a particular function. For example, multipotent blood stem cells give rise to the red cells, white cells and platelets in the blood. Importantly, totipotent cells must be able to differentiate not only into any cell in the organism, but also into the extraembryonic tissue associated with that organism. For example, human stem cells are considered totipotent only if they can develop into any cell in the body, or into placental cells that do not become part of the developing fetus. This fact is an important aspect of the stem cell controversy because the human embryonic stem cells used for research purposes are pluripotent; they are collected from human embryos that have developed past the totipotent cell stage. All human embryos used in stem cell experimentation are destroyed in the process. |
|
|
blast cells p1022
|
Blast cells are immature precursors of either lymphocytes (lymphoblasts), or granulocytes (myeloblasts). They do not normally appear in peripheral blood. When they do, they can be recognized by their large size, and primitive nuclei (ie the nuclei contain nucleoli), as in the picture. When present in the blood, they often signify ACUTE LEUKEMIA. This particular case demonstrates the presence of an Auer Rod, which is pathognomonic for Acute Myeloid Leukemia. Otherwise, special stains and surface marker techniques are needed to identify the lineage of the cells.
|
|
|
Methemoglobin
|
is a form of the oxygen-carrying protein hemoglobin (British English: haemoglobin), in which the iron in the heme group is in the Fe3+ state, not the Fe2+ of normal hemoglobin. Methemoglobin cannot carry oxygen. It is a bluish chocolate-brown in color. The NADH-dependent enzyme methemoglobin reductase (diaphorase I) is responsible for converting methemoglobin back to hemoglobin.
Normally one to two percent of people's hemoglobin is methemoglobin; a higher percentage than this can be genetic or caused by exposure to various chemicals and depending on the level can cause health problems known as Methemoglobinemia. A higher level of methemoglobin will tend to cause a pulse oximeter to read closer to 85% regardless of the true level of oxygen saturation.[1] |
|
|
Anemia p927-948
|
is a decrease in normal number of red blood cells (RBCs) or less than the normal quantity of hemoglobin in the blood.[1][2] However, it can include decreased oxygen-binding ability of each hemoglobin molecule due to deformity or lack in numerical development as in some other types of hemoglobin deficiency.
Since hemoglobin (found inside RBCs) normally carries oxygen from the lungs to the tissues, anemia leads to hypoxia (lack of oxygen) in organs. Since all human cells depend on oxygen for survival, varying degrees of anemia can have a wide range of clinical consequences. The three main classes of anemia include excessive blood loss (acutely such as a hemorrhage or chronically through low-volume loss), excessive blood cell destruction (hemolysis) or deficient red blood cell production (ineffective hematopoiesis). Anemia is the most common disorder of the blood. There are several kinds of anemia, produced by a variety of underlying causes. Anemia can be classified in a variety of ways, based on the morphology of RBCs, underlying etiologic mechanisms, and discernible clinical spectra, to mention a few. There are two major approaches: the "kinetic" approach which involves evaluating production, destruction and loss[3], and the "morphologic" approach which groups anemia by red blood cell size. The morphologic approach uses a quickly available and cheap lab test as its starting point (the MCV). On the other hand, focusing early on the question of production may allow the clinician more rapidly to expose cases where multiple causes of anemia coexist. |
|
|
Normochromic p938-948
|
anemia in which the hemoglobin content of the red cells as measured by the MCHC is in the normal range.
|
|
|
Macrocytic p931-933
|
in which the erythrocytes ("red blood cells" or RBCs) are larger than their normal volume. The normal RBC volume in humans is about 80 to 100 femtoliters (fL= 10-15 L). In slightly less correct metric terminology which does not use standard volume units, the size may be given in equivalent cubic micrometres (1 μm3 = 1 fL). The condition of having red cells which are on average too large, is called macrocytosis.
In a macrocytic anemia the larger red cells are always associated with insufficient numbers of cells and often also insufficient hemoglobin content per cell, both factors which more than make up for the larger cell size, to produce a total blood hemoglobin concentration deficiency. Macrocytic anemia is not a disease, but a condition: a general classification of a set of pathologies. Many specific pathologies are known which result in macrocytic-type anemias, but which produce slightly different sets of appearances, some of which are detectable from red and white cell mophology, and others only from chemical tests on the blood. |
|
|
Polycythemia p948-950
|
is a condition in which there is an increase in the proportion of blood volume that is occupied by red blood cells, which is measured as hematocrit level.
It can be due to an increase in the mass of red blood cells |
|
|
Normocytic p938-948
|
is a common issue that occurs for men and women typically over 85 years old. Its prevalence increases with age, reaching 44 percent in men older than 85 years.[1] Normocytic anemia is the most frequently encountered type of anemia
|
|
|
Microcytic p1002
|
s a generic term for any type of anemia characterized by small red blood cells. The normal mean corpuscular volume (abbreviated to MCV on full blood count results) is 76-100 fl, with smaller cells (<76 fl) described as microcytic and larger cells (>100 fl) as macrocytic.
In microcytic anemia, the red blood cells (erythrocytes) are usually also hypochromic, meaning that the red blood cells are paler than usual. This can be quantified as the mean corpuscular hemoglobin or mean cell hemoglobin (MCH), the amount of hemoglobin per cell; the normal value is 27-32 picograms (pg). Similar is the mean corpuscular hemoglobin concentration or mean cell hemoglobin concentration (MCHC), giving the amount of hemoglobin per volume of erythrocytes (normally about 320-360 g/l or 32-36 g/dl). Typically, therefore, anemia of this category is described as "microcytic, hypochromic anemia". |
|
|
causes microcytic anemia
|
* childhood
o iron deficiency anemia,[1] by far the most common cause of anemia in general and of microcytic anemia in particular o thalassemia * adulthood o iron deficiency anemia o sideroblastic anemia, congenital or acquired o sometimes, anemia of chronic disease, although this more typically causes normochromic, normocytic anemia o lead poisoning (rare) o pyridoxine deficiency Other causes that are "typically" thought of as causing normocytic anemia or macrocytic anemia must also be considered, and the presence of two or more causes of anemia can distort the "typical" picture of each. |
|
|
normocytic anemia causes
|
* a decreased production of normal-sized red blood cells (e.g., anemia of chronic disease, aplastic anemia);
* an increased destruction or loss of red blood cells (e.g., hemolysis, posthemorrhagic anemia); * an uncompensated increase in plasma volume (e.g., pregnancy, fluid overload); * or a mixture of conditions producing microcytic and macrocytic anemia |
|
|
Leukocytosis p955
|
a raised white blood cell count (the leukocyte count) above the normal range. This increase in leukocytes (primarily neutrophils) is usually accompanied by a "left shift" in the ratio of immature to mature neutrophils. The increase in immature leukocytes increases due to proliferation and release of granulocyte and monocyte precursors in the bone marrow which is stimulated by several products of inflammation including C3a and G-CSF.
Although it may indicate illness, leukocytosis is considered a laboratory finding instead of a separate disease. This classification is similar to that of fever, which is also a test result instead of a disease. A leukocyte count above 25 to 30 x 109/L is termed a leukemoid reaction, which is the reaction of a healthy bone marrow to extreme stress, trauma, or infection. (It is different from leukemia and from leukoerythroblastosis, in which immature blood cells are present in peripheral blood.) |
|
|
Leukemia p960-967
|
is a cancer of the blood or bone marrow and is characterized by an abnormal proliferation (production by multiplication) of blood cells, usually white blood cells (leukocytes). Leukemia is a broad term covering a spectrum of diseases. In turn, it is part of the even broader group of diseases called hematological neoplasms.
|
|
|
Thrombus p991
|
is the final product of the blood coagulation step in hemostasis. It is achieved via the aggregation of platelets that form a platelet plug, and the activation of the humoral coagulation system (i.e. clotting factors). A thrombus is normal in cases of injury, but pathologic in instances of thrombosis.
|
|
|
Plasmin p184
|
is an important enzyme (EC 3.4.21.7) present in blood that degrades many blood plasma proteins, most notable, fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein is encoded by the PLG gene.
|
|
|
Leukopenia p955
|
is a decrease in the number of white blood cells (leukocytes) found in the blood, which places individuals at increased risk of infection.
Neutropenia is a sub-type of leukopenia that refers to a decrease in the number of circulating neutrophil granulocytes, the most abundant white blood cells. The terms leukopenia and neutropenia may occasionally be used interchangeably, as the neutrophil count is the most important indicator of infection risk. |
|
|
Granulocytosis p956
|
s the presence in peripheral blood of an increased number of granulocytes, a category of white blood cells. Often, the word refers to an increased neutrophil granulocyte count, as neutrophils are the main granulocytes.
|
|
|
Embolus p991
|
occurs when an object (the embolus, plural emboli; from the Greek ἔμβολος "clot, lit. ram") migrates from one part of the body (through circulation) and causes a blockage (occlusion) of a blood vessel in another part of the body. The term was coined in 1848 by Rudolph Carl Virchow.[1] This is in contrast with a thrombus, or clot, which forms at the blockage point within a blood vessel and is not carried from somewhere else.
|
|
|
leukocytosis causes
|
is very common in acutely ill patients. It occurs in response to a wide variety of conditions, including viral, bacterial, fungal, or parasitic infection, cancer, hemorrhage, and exposure to certain medications or chemicals including steroids. Leukocytosis can also be the first indication of neoplastic growth of leukocytes.
For lung diseases such as pneumonia and tuberculosis, WBC count is very important for the diagnosis of the disease, as leukocytosis is usually present. The mechanism that causes leukocytosis can be of several forms: an increased release of leukocytes from bone marrow storage pools, decreased margination of leukocytes onto vessel walls, decreased extravasation of leukocytes from the vessels into tissues, or an increase in number of precursor cells in the marrow. Certain medications, including corticosteroids, lithium and beta agonists, may cause leukocytosis. |
|
|
patho of a thrombus
|
Specifically, a thrombus is the inappropriate activation of the hemostatic process in an uninjured or slightly injured vessel. A thrombus in a large blood vessel (mural thrombus) will decrease blood flow through that vessel. In a small blood vessel (occlusive thrombus), blood flow may be completely cut-off resulting in death of tissue supplied by that vessel. If a thrombus dislodges and becomes free-floating, it is termed as an embolus.
Some of the conditions which elevate risk of blood clots developing include atrial fibrillation (a form of cardiac arrhythmia), heart valve replacement, a recent heart attack, extended periods of inactivity (see deep venous thrombosis), and genetic or disease-related deficiencies in the blood's clotting abilities. |
|
|
leukopenia causes
|
Low white cell counts are associated with chemotherapy, radiation therapy, leukemia (as malignant cells overwhelm the bone marrow), myelofibrosis and aplastic anemia (failure of white and red cell creation, along with poor platelet production). In addition, many common medications can cause leukopenia (see below).
Other causes of low white blood cell count include: Influenza, systemic lupus erythematosus, Hodgkin's lymphoma, some types of cancer, typhoid, malaria, tuberculosis, dengue, Rickettsial infections, enlargement of the spleen, folate deficiencies, psittacosis and sepsis. Many other causes exist, such as a deficiency in certain minerals such as copper and zinc. Pseudoleukopenia can develop upon the onset of infection. The leukocytes (predominately Neutrophils, responding to injury first) are marginalized in the blood vessels so that they can scan for the site of infection. This means that even though there is increased WBC production, it will appear as though it is low from a blood sample, since the blood sample is of core blood and does not include the marginalized leukocytes. |
|
|
granulocytosis causes p956
|
An increase in eosinophil granulocyte is known as eosinophilia.
Granulocytosis can be a feature of a number of diseases: * Infection, especially bacterial * Malignancy, most notably leukemia (it is the main feature of chronic myelogenous leukemia, CML) * Autoimmune disease |
|
|
Automaticity p1041
|
the capacity of a cell to initiate an impulse without an external stimulus
|
|
|
Excitability
|
Capable of responding to stimuli
|
|
|
Inotropic p1050
|
is an agent that alters the force or energy of muscular contractions. Negatively inotropic agents weaken the force of muscular contractions. Positively inotropic agents increase the strength of muscular contraction.
Most commonly, the inotropic state is used in reference to various drugs that affect the strength of contraction of heart muscle (myocardial contractility). However, it can also refer to pathological conditions. For example, enlarged heart muscle (ventricular hypertrophy) can increase inotropic state, while dead heart muscle (myocardial infarction) can decrease it. |
|
|
Preload p1046
|
is the pressure stretching the ventricle of the heart,[1] after passive filling of the ventricle and subsequent atrial contraction. If the chamber is not mentioned, it is usually assumed to be the left ventricle.
Preload is theoretically most accurately described as the initial stretching of a single cardiac myocyte prior to contraction. This cannot be measured in vivo and therefore other measurements are used as estimates. Estimation is inaccurate, for example in a chronically dilated ventricle new sarcomeres may have formed in the heart muscle allowing the relaxed ventricle to appear enlarged. The term end-diastolic volume is better suited to the clinic, although not exactly equivalent to the laboratory term preload. |
|
|
Contractility p1658
|
the intrinsic ability of a cardiac muscle fibre to contract at a given fibre length. Changes in the ability to produce force during contraction result from different degrees of binding between myosin (thick) and actin (thin) filaments. The degree of binding that occurs depends on concentration of calcium ions in the cell; in an intact heart, it is usually the action of the sympathetic nervous system (through catecholamines) that determines the concentration of calcium ions in the cytosol of cardiac muscle cells. All factors that cause an increase in contractility work by causing an increase in intracellular [Ca++] during contraction
|
|
|
systole
|
hase of the cardiac cycle where the myocardium is contracting in a coordinated manner in response to an endogenous electrical stimulus, and pressure is being generated within the chambers of the heart driving blood flow. Experimental and clinical measurement of systolic contraction are often based on ejection fraction and cardiac output. The chamber most often discussed is the left ventricle. However, all four chambers of the heart undergo systole and diastole in a timed fashion so that blood is propelled forward through the cardiovascular system.
|
|
|
pulse pressure
|
is the difference between systolic and diastolic blood pressure, or the change in blood pressure seen during a contraction of the heart.
|
|
|
Rhythmicity p1041
|
s the spontaneous depolarization and repolarization event that occurs in a repetitive and stable manner within the cardiac muscle. Rhythmicity is often abnormal or lost in cases of cardiac dysfunction or cardiac failure.
|
|
|
Conductivity
|
The electrical conduction system that controls the heart rate. This system generates electrical impulses and conducts them throughout the muscle of the heart, stimulating the heart to contract and pump blood.
Among the major elements in the cardiac conduction system are the sinus node, atrioventricular node, and the autonomic nervous system. |
|
|
Chronotropic
|
effects (from chrono-, meaning time) are those that change the heart rate.
|
|
|
Afterload p1046, 1048
|
s the pressure stretching the ventricle of the heart,[1] after passive filling of the ventricle and subsequent atrial contraction. If the chamber is not mentioned, it is usually assumed to be the left ventricle.
Preload is theoretically most accurately described as the initial stretching of a single cardiac myocyte prior to contraction. This cannot be measured in vivo and therefore other measurements are used as estimates. Estimation is inaccurate, for example in a chronically dilated ventricle new sarcomeres may have formed in the heart muscle allowing the relaxed ventricle to appear enlarged. The term end-diastolic volume is better suited to the clinic, although not exactly equivalent to the laboratory term preload. |
|
|
Syncytium p282
|
is a large cell-like structure filled with cytoplasm containing many nuclei. Most cells in all organisms have a single nucleus; syncytia are specialized forms used by various organisms in normal tissue.
|
|
|
Diastole p1033
|
the period of time when the heart fills with blood after systole (contraction). Ballistics accurately describes Diastole as recoil opposed to coil or Systole. Ventricular diastole is the period during which the ventricles are relaxing, while atrial diastole is the period during which the atria are relaxing
|
|
|
mean arterial pressure p1061
|
term used in medicine to describe an average blood pressure in an individual It is defined as the average arterial pressure during a single cardiac cycle.
Mean arterial pressure can be determined from:[2] MAP = (CO \times SVR) + CVP where: * CO is cardiac output * SVR is systemic vascular resistance * CVP is central venous pressure and usually small enough to be neglected in this formula. |
|
|
Clinical manifestations of cancer p385-389
|
Clinical manifestations of cancer
|
|
|
Pain and cancer p385-387
|
pain generally is associated with the late stages of cancer. It can be caused by pressure, obstruction, invasion of a structure sensitive to pain, stretching, tissue destruction, and inflammation.
|
|
|
Fatigue and cancer p387
|
the most frequently reported symptom of cancer and cancer treatment
|
|
|
Cachexia p387-388
|
loss of appetite, early satiety, weakness, inability to maintain weight, taste alteration, altered metabolism) leads to protein-calorie malnutrition, and progressive wasting
|
|
|
Cancer and anemia
|
usually occurs because of malnutrition, chronic bleeding and resultant iron deficiency, chemotherapy, radiation and malignancies in the blood-forming organs.
|
|
|
Leukopenia and cancer
|
usually a result of chemotherapy (which is toxic to the bone-marrow) or radiation (which kills circulating leukocytes).
|
|
|
Thrombocytopenia and cancer p981-983
|
usually the result of chemotherapy or malignancy in the bone marrow.
|
|
|
Infection and cancer
|
may be caused by leukopenia, immunosupression, or debility associated with advanced disease. It is the most significant cause of complication and death.
|
|
|
Mechanistic framework of symptom onset
|
mediated by cytokines acting on the peripheral and central nervous system
|
|
|
Paraneoplastic syndromes p388-389, 1240
|
symptom complexes that cannot be explained by the local or distant spread of the tumor or by hormones released by the tissue from which the tumor arose.
|
|
|
Cancer treatment
|
surgery, radiation therapy, chemotherapy, immunotherapy, and combination of these modalities.
|
|
|
Theoretic basis of chemotherapy- p389-393
|
- the vulnerability of tumor cells in various stages of the cell cycle. The goal of chemotherapy is to eradicate enough tumor cells so that the body’s natural defenses can eradicate remaining cells
|
|
|
Ionizing radiation p71-72
|
causes cell damage, so that the goal of radiation therapy is to damage the tumor without causing excessive toxicity or damage to undismayed structures.
|
|
|
Surgical theory
|
is used for nonmetastatic disease for which cure is possible by removing the tumor and as a palliative measure to alleviate symptoms
|
|
|
Immunotherapy p393-396
|
appropriate for cancers that cannot be effectively managed by chemotherapy or radiation, usually because enough tumor cells are inactive and vulnerable to these modalities.
|
|
|
Forms of immunotherapy
|
one kind known as biologic response modifiers include immunomodulating agents, interferons, anitgens, effector cells, lymphokines, and monoclonal antibodies.
|
|
|
Effector cells and lymphokines p395-396
|
provide a form of cellular immunotherapy that involves the transfer of cytotoxic T cells (Tc cells) that are specific for tumor cell antigens
|
|
|
Immunomodulating agents p394-395
|
provide nonspecific stimulation of the immune system by means of an adjuvant; they are most effective in treating skin cancers.
|
|
|
Antigens p395
|
cause regression in skin tumors by producing a hypersensitivity response that affects the antigenic properties of the cell surface.
|
|
|
Monoclonal antibodies p396-398
|
ultimately may be used both as diagnostic reagents for detecting cancer and a form of cancer therapy in which antibodies specific for tumor antigens would mediate tumor rejection.
|
|
|
key to increasing compliance in cancer treatment
|
appropriate education about the side effects and treatments
|
|
|
Side effects
|
directly related to the targeting of the rapidly growing cell
|
|
|
Chemotherapy and radiation side effects
|
may cause an increased cell turnover leading to oral ulcers malabsorbtion and diarrhea.
|
|
|
Disruption of barrier defenses in the GI tact
|
Disruption of barrier defenses in the GI tact
|
|
|
Nausea
|
thought to be caused by an agents direct action on the vomiting center in the central nervous system. Thus aggressive treatment with antiemetic theory is mandated
|
|
|
Chemotherapy and bone marrow suppression
|
can be caused in all three cell lines, red, white and platelets. Anemia is common with red cell suppression, decreased platelet numbers can increase bleeding and decreased white blood cells increases the risk of infection
|
|
|
Hair loss
|
results from chemotherapy effects on hair follicles. Alopecia is usually temporary and not all agents cause it.
|
|
|
Radiation and chemotherapy effects on gametes
|
leading to varying degrees of decreased fertility and premature menopause. These effects are dose- and age-dependent, with the prepubertal gonad thought to be more resistant to damage
|
|
|
Craniospinal irradiation for cns tumors
|
may affect the hypothalamus or pituitary gland resulting in gonadal failure.
|
|
|
Describe how monoclonal antibodies assist in the diagnosis and treatment of cancer.
|
One possible treatment for cancer involves monoclonal antibodies that bind only to cancer cell-specific antigens and induce an immunological response against the target cancer cell. Such mAb could also be modified for delivery of a toxin, radioisotope, cytokine or other active conjugate; it is also possible to design bispecific antibodies that can bind with their Fab regions both to target antigen and to a conjugate or effector cell. In fact, every intact antibody can bind to cell receptors or other proteins with its Fc region.
|
|
|
what is this image
|
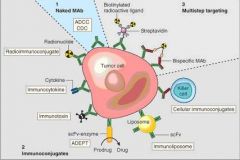
|
|
|
Blood
|
consists of a variety of formed elements about 90% water and 10% solutes. In adults the total blood volume is approximately 5.5L
|
|
|
Plasma
|
complex aqueous liquid, contains three major groups of plasma proteins: albumins, globulins, and clotting factors
|
|
|
Cellular elements of blood
|
erythrocytes, leukocytes, lymphocytes and platelets
|
|
|
Erythrocytes p895-896
|
- the most abundant cells of the blood, occupying approximately 48% of the blood volume in men and 42% in women. Responsible for tissue oxygenation
|
|
|
Leukocytes
|
fewer in number than erythrocytes and constitute approx. 5000-10000 cells per mm3 of blood. Leukocytes defend the body against infection and remove dead or injured host cells.
|
|
|
Classification of leukocyte
|
classified as with granulocytes (neutrophils, basophils, eosinophils) or agranulocytes (monocytes, macrophages and lymphocytes)
|
|
|
Natural killer cells
|
- resemble lymphocyte, kill some types of virus infected cells without being exposed to them beforehand, and tumor cells in vitro
|
|
|
Platelets are not cells
|
- they are disk-shaped cytoplasmic fragments. Platelets are essential for blood coagulation and control of bleeding.
|
|
|
Lymphoid organs p898-900
|
primary ( thymus and bone marrow) and secondary ( spleen, lymph nodes, tonsils, and Peyer patches of the small intestine) These organs are sites of residence, proliferation, differentiation or function of lymphocytes and mononuclear phagocytes
|
|
|
Spleen p898-899
|
- the largest of the secondary lymphoid organs and functions as the site of fetal hematopoiesis, filters and cleanses the blood and is a reservoir for lymphocytes and other blood cells.
|
|
|
Lymph nodes p899-900
|
are the site of development or activity of large numbers of lymphocytes, monocytes and macrophages
|
|
|
Mononuclear Phagocyte system (MPS)-
|
previously called with reticuloendothial system (RES) composed of monoblasts, promonocytes and monocytes in bone marrow, monocytes in peripheral blood and macrophages in tissue.
|
|
|
MP
|
the main line of defense against bacteria in the bloodstream, and cleanses he blood by removing old, injured, or dead blood cells; antigen-antibody complexes: and macromolecules
|
|
|
Hematopoiesis p900-904
|
blood cell production occurs in the liver and spleen of the fetus and in the bone marrow after birth. Continues throughout life to replace blood cells that grow old or die are killed by disease or are lost through bleeding.
|
|
|
Two stages of hematopoiesis
|
proliferation and differentiation or maturation. Each type of blood cell has parent cells called stem cells
|
|
|
Regulation of hematopoiesis
|
possibly occurs two ways- by stromal cells involved in cell contact processes and by cytokines and or regulatory molecules
|
|
|
Humoral colony-stimulating factors (CFS’s)
|
necessary for the adequate growth of myeloid, erythroid, lymphoid and megakaryocytic lineages
|
|
|
Bone marrow p904
|
consists of blood vessels, nerves, mononuclear phagocytes, stem cells, blood cells in various stages of differentiation, stromal cells and fatty tissue.
|
|
|
Hemoglobin
|
enables the blood to transport 100 times more oxygen than could be transported dissolved in plasma alone
|
|
|
Erythropoiesis p909
|
on the presence of vitamins (especially vit. B12 folate riboflavin panothenic acid niacin ascorbic acid and vit E
|
|
|
Regulation of Erythropoiesis
|
mediated by erythropeietin, hypoxia and causes a compensatory increase in erythrocyte production if the oxygen content of the blood decreases because of anemia, high altitude, or pulmonary disease.
|
|
|
Maintenance of optimal levels of granulocytes and monocytes in the blood-
|
depends on the availability of pluripotential stem cells in the marrow, induction of these into committed stems cells and timely release of new cells from the marrow.
|
|
|
How platelets develop from megakaryocytes
|
a process called domitosis In endomitosis the megakaryocytes undergo mitosis but not cytokinesis; thus the cell does not divide into daughter cells.
|
|
|
Platelet activation
|
the inflammatory response.
|
|
|
Blood clot
|
- a meshwork of protein strands that stabilizes the platelet plug. The strands are made of fibrin. Fibrin is the end product of the coagulation cascade.
|
|
|
Classical view of coagulation p980-993
|
the coagulation cascade is comprised of both intrinsic and extrinsic pathways. It is now known that the tissue factor (TF) is the primary cellular imitator of blood coagulation after vessel injury. The TF-FVIIa complex activated the coagulation cascade.
|
|
|
Hemostasis or the arrest of bleeding
|
- involves (a) vasoconstriction, (vasoplasm) (b) formation of a platelet plug, (c) activation of the clotting cascade, (d) formation of a blood clot and (e) clot retraction and clot dissolution
|
|
|
Platelet activation
|
involves four separate processes (1) adhesion (2) aggregation (3) secretion (4) procoagulation activity.
|
|
|
Prevention of clots
|
several anticoagulant mechanisms exist including antithrombin-heparan mechanism, the tissue factor pathway inhibitor mechanism, and the protein C anticoagulant pathway.
|
|
|
Lysis of blood clots p918-919
|
the function of the fibrinolytic system. Plasmin is a degrading enzyme of fibrin clots. It is released as plasminogen and activated by tissue plasminogen activator (t-PA) thrombin, fibrin and factor XII.
|
|
|
Bone marrow specimen p920
|
cells are assessed with respect to (1) relative numbers of stem cells and their developing daughter cells and (2) morphologic structure
|
|
|
Blood composition and age
|
little changes with age. A delay in erythrocyte replenishment may occur after bleeding presumably because of iron deficiency
|
|
|
Lymphocyte function and age
|
appears to decrease with age. Particularly affected is a disease in cellular immunity.
|
|
|
Anemia p927-948
|
reduction in the number or volume if circulating RBC’s or an alteration in hemoglobin. Polycythemias are excessive RBC’s or volume
|
|
|
Classification of anemia’s-
|
(a) erythrocyte size or concentration of hemoglobin (b) cause of low blood count (c) the kinetics of why constant and adequate numbers of mature erythrocytes are not maintained in the circulation
|
|
|
Clinical manifestations of anemia
|
may be demonstrated in all organs and tissues throughout the body. Decreased oxygen delivery to tissue causes fatigue, dyspnea, syncope, angina, compensatory tachycardia, and organ dysfunction
|
|
|
Macrocytic (or megoblastic) normochromic anemias
|
characterized by larger than normal RBC’s with smaller than normal nuclei.
|
|
|
Causes of macrocytic anemias
|
caused most commonly by deficiency of vit b12 (caused by lack of intrinsic factor [IF] or folate. In pernicious anemia this can be fatal unless replaced. Done by injection or sometimes larger than normal doses of vit b12 orally may be effective
|
|
|
Folate deficiency anemia
|
caused by inadequate dietary intake. After tx with replacement therapy no further treatment is required.
|
|
|
Microcytic-hypochromic anemias
|
characterized by abnormally small RBC’s with insufficient hbg content. The most common cause is iron deficiency
|
|
|
Iron deficiency anemia
|
most common type of anemia worldwide. It usually develops slowly, with gradual insidious onset of symptoms. Fatigue, weakness, dyspnea, alteration of various epithelial tissues, and vague neuromuscular complaints result
|
|
|
Iron deficiency anemia causes
|
usually a result of blood loss or poor nutritional intake. Individuals at highest risk for developing IDA are the elderly, females, infants, and those living in poverty. Anemia is also recognized as part of the nonspecific acute phase response to any type of inflammation. Once the source of blood loss is identified and corrected, oral iron replacement therapy can be initiated.
|
|
|
Elevated reticulocyte count-
|
good index of response to iron therapy. A recently discovered indicator of iron levels is serum transferring receptor (sTfR)
|
|
|
Sideroblastic anemia (SA) p936-938
|
results from impaired iron metabolism resulting in dysfunctional hbg synthesis that produces abnormal cellular sequestration of iron. SA’s may be hereditary or acquired, and treatment varies depending on the cause.
|
|
|
Normacytic-normochromic
|
anemias are characterized by insufficient numbers of normal erythrocytes. Included in this category are aplastic, posthemorrhagic and hemolytic anemias and anemia of chronic disease.
|
|
|
Aplastic anemia
|
critical condition characterized by pancytopenia or a reduction or absence of all three blood cell types. Unless the cause is determined bone marrow aplasia results in death.
|
|
|
Acute blood loss from hemorrhage
|
- results in normocytic-normochromic anemia (posthemorrhagic anemia). Restoration of blood volume by plasma expanders or transfusion may diminish subjective symptoms of anemia. Hbg restoration may take 6-8 weeks.
|
|
|
Hemolytic anemia
|
premature destruction of erythrocytes may be acquired or hereditary. Of the acquired forms, autoimmune reaction (immunohemolytic) and drug-induced hemolysis are the most common
|
|
|
Types of immunohemolytic anemias
|
(1) warm antibody type (2) cold agglutinin type (3) cold hemolysins
|
|
|
Failure of Erythropoiesis in anemia of chronic disease
|
may result in release of cytokines and lactoferrin by phagocytic cells. Lactoferrin has been shown to cause abrupt diseases in plasma iron levels by interfering with the normal iron cycle.
|
|
|
Anemia of chronic disease (ACD)
|
mild to moderate anemia in individuals with chronic conditions that include AIDS, renal failure, and malignancies. ACD is one of the most common conditions encountered in medicine.
|
|
|
The Th1 response
|
appears to be the common factor among the various inflammatory and infectious conditions associated with ACD
|
|
|
Mechanisms associated with ACD
|
– (1) decreased erythrocyte lifespan (2) ineffective bone marrow response to erythropoietin (3) altered iron metabolism
|
|
|
Polycythemia vera
|
characterized by excessive proliferation of erythrocyte precursors in the bone marrow. Signs and symptoms result directly from increased blood volume and viscosity.
|
|
|
Treatment for decreasing excessive RBC population-
|
- therapeutic phlebotomy to remove excessive blood volume and use radioactive phosphorous have been helpful.
|
|
|
shift to the left
|
a term used in medicine in connection with the white blood cells (neutrophils) that generally increase in their numbers and in their precursor more primitive forms in many disease processes - bacterial infections, inflammations, etc. In more serious medical conditions, the spectrum of neutrophils from mature through their precursor forms ranges in sequence from mature neutrophil, segmented neutrophil, band neutrophil, metamyelocyte, myelocyte and myeloblast, the last of these being most common in (malignant) myeloblastic leukaemia. The "shift to the left" then - in medicine at least - indicates the increased presence of these less mature forms relative to their mature ones.
|
|
|
Leukocytosis
|
- caused by the pathologic conditions, such as malignancies and hematologic disorders
|
|
|
Granulocytopenia p956
|
condition resulting in a severe decrease in neutrophils can be a life threatening condition if sepsis occurs; often it is caused by chemotherapeutic agents, severe infection, and radiation
|
|
|
Eosinophilia p956-958
|
results most commonly from parasitic invasion and ingestion or inhalation of toxic foreign particles.
|
|
|
Monocytosis p958
|
occurs during the late or recuperative phase of infection when macrophages (mature monocytes) phagocytose surviving microorganisms and debris
|
|
|
Infectious mononucleosis p958-960
|
self limiting, nonneoplastic, lymphoproliferate syndrome caused by infection of B cells, most commonly the Epstein-Barr (EBV) a herpes type virus. Most commonly affects young adults between 15-35 years of age who have not previously had EBV infection during childhood. Most cases start with fever and sore throat a temperature elevation 7-10 days, enlargement and tenderness of the cervical lymph nodes from inflammation at the site of viral entry.
|
|
|
Common pathological feature of leukemia
|
uncontrolled proliferation of leukocytes
|
|
|
Three designations of leukemias
|
(a) lymphocytic (b) myelocytic or myelogenous (c) moncytic
|
|
|
Two types of acute leukemias
|
(a) acute myelogenous leukemia (AML) (b) acute lymphocytic leukemia (ALL)
|
|
|
Two principle types of chronic leukemias
|
chronic myelocytic leukemia and chronic lymphocytic leukemia
|
|
|
Cause of leukemia
|
a clonal disorder. High incidence of acute leukemias and CLL is reported in certain families, suggesting a genetic predisposition.
|
|
|
Philadelphia chromosome p962
|
the most common genetic abnormality in adult Acute lymphatic leukemia and chronic myelocytic leukemia
|
|
|
Mutation in a third of patients with AML
|
mutation in the receptor tyrosine kinase FLT3
|
|
|
Leukemia and blast cells
|
these “crowd out” the marrow and cause cellular proliferation of the other cell lines to cease.
|
|
|
Acute leukemia and cells
|
- involves abnormal proliferation of precursor cells, decreased rate of apoptosis, and an arrest of differentiation.
|
|
|
Chronic lymphocytic leukemia
|
involves transformation and accumulation of monoclonal B lymphocytes. Accumulation is the result of cell cycle arrest in the G0/G1 phase creating B cells resistant to apoptosis
|
|
|
Clinical manifestations of leukemia
|
fatigue caused by anemia, bleeding caused by thrombocytopenia, fever secondary to infection, anorexia, and weight loss
|
|
|
Treatment of choice for leukemia
|
chemotherapy.
|
|
|
Life expectancy for leukemias-
|
leukemias are associated with 20%-50% long term survival rate. Chronic leukemias are associated with a longer life expectancy.
|
|
|
Myeloma p967-971
|
a neoplasm of immunocytes called plasma cells. The tumor may be solitary or multifocal, known as multiple myeloma.
|
|
|
Multiple myeloma
|
is a disease of transformed precursor plasma cells that have completed rearrangement of immunoglobin heavy chain in the lymph nor or transformed fully matured (differentiated) plasma cells in the bone marrow.
|
|
|
Cause of multiple myeloma
|
unknown- genetic factors and chronic stimulation of the mononuclear phagocyte system by bacteria, viral agents, and chemicals have been suggested
|
|
|
Most common alteration in multiple myeloma
|
chromosome 13 abnormality, 86%
|
|
|
Major clinical manifestation multiple myeloma
|
recurrent infections caused by suppression of the humoral immune response and renal disease such as Bence-Jones protenuria
|
|
|
Treatments multiple myeloma
|
chemo, stem cell transplants, thalidomide
|
|
|
Effect on lymphocytes in acute infections and immune deficiency syndromes-
|
decreased (lymphocytopenia) and occurs in viral infections, infectious mono, infectious hepatitis, leukemia, lymphomas, and some chronic infections
|
|
|
Lymphomas p336
|
are tumors of primary lymphoid tissue (thymus and bone marrow) or secondary tissue ( lymph nodes, spleen, tonsils, and intestinal lymphoid tissue) the major tyoes are Hodgkin’s and non-Hodgkin’s
|
|
|
Reed-Sternberg cell
|
the name of the cells that have distinctive abnormal chromosomes are present in multiple cells of the lymph nodes of an individual with Hodgkin’s lymphoma.
|
|
|
Pathogenesis of Hodgkin’s lymphoma p973-975
|
viruses are suspected. Some familial clustering suggests an unknown genetic mechanism.
|
|
|
Initial sign of Hodgkin’s
|
an enlarged unilateral painless mass or swelling, most commonly in the neck. Local symptoms are caused by pressure or obstruction
|
|
|
Treatment of Hodgkin’s lymphoma
|
radiation therapy and chemo. Cure is possible regardless of the stage of Hodgkin lymphoma: however, individuals treated with chemo who relapse less than 2 years have a poor prognosis
|
|
|
Cause of lymph node enlargement and cancerous transformation in non-Hodgkin’s p975-978
|
Unknown. Immunosupressed persons have a greater incidence of non-Hodgkin lymphoma suggesting an immune mechanism. The swelling of these nodes is usually painless and occurs over time.
|
|
|
Survival and treatment of non-Hodgkin’s lymphoma
|
survival is for long periods and the treatment consists of chemo.
|
|
|
Burkitt lymphoma p978
|
involves the jaw and facial bones and occurs in children from east-central Africa and New Guinea
|
|
|
Splenomegaly p979
|
not necessarily considered pathologic but may indicate underlying pathology. Results from a wide variety of conditions, most notably those caused by acute inflammatory or infectious processes or those that produce splenic congestion or infiltration.
|
|
|
Hypersplenism p979
|
associated with splenomegaly and pancytopenia
|
|
|
Thrombocytopenia
|
characterized by a platelet count below 100,000. a count below 50,000 increases the potential for hemorrhage associated with minor trauma. Thrombocytopenia exists n primary and secondary forms
|
|
|
Secondary thrombocytopenia
|
commonly associated with autoimmune diseases and viral infections; bacterial sepsis, which may cause disseminated intravascular coagulation (DIC), also result in thrombocytopenia
|
|
|
Heparin
|
thrombocytopenia- develops in approx. 2-155 of individuals receiving heparin
|
|
|
ITP
|
a major cause of platelet destruction, often affecting females, and results in hemorrhaging that ranges from minor development of petechiae to major bleeding from mucosal site.
|
|
|
TTP
|
causes platelet aggregation leading to microcirculatory occlusion
|
|
|
Thrombocythemia
|
characterized by a platelet count greater than 600,000 of blood and is symptomatic when the count exceeds 1,000,000 and when the risk for intravascular clotting (thrombosis) is high
|
|
|
Primary throbocythemia
|
caused by accelerated platelet production in the bone marrow characterized by hyperplasia of megakaryocytes
|
|
|
Alterations in normal platelet adherence or aggregation
|
prevent platelet plug formation and may result in prolonged bleeding times
|
|
|
Qualitative platelet dysfunction-
|
results from disorders f platelet adhesion, platelet aggregation, platelet secretion and procoagulant activity.
|
|
|
Disorders of coagulation
|
usually are caused by defects or deficiencies of one or more of the clotting factors.
|
|
|
Impaired coagulation
|
when there is a deficiency of vitamin K because of insufficient hepatic production of prothrombin and clotting factors II, VII, IX, and X
|
|
|
Liver disease and alterations of coagulation
|
accounts for pattern of hemostatic derangement caused by a disruption of the synthesis of clotting factors.
|
|
|
Thromboembolic disease
|
- results from a fixed (thrombus) or moving (embolism) clot that obstructs blood flow within a vessel, denying nutrients to tissue distal to the occlusion, death can result when clots are lodged in the heart, brain or lungs
|
|
|
Hypercoagulability
|
result of deficient anticoagulation proteins. Secondary causes are conditions that promote venous stasis.
|
|
|
Triad of Virchow
|
refers to three factors that can cause a thrombus formation: (1) loss of integrity of the vessel wall, (2) abnormalities of blood flow, and (3) alterations in the blood constituents.
|
|
|
DIC
|
is complex syndrome resulting from a variety of clinical conditions that cause release of tissue factor and results in activation and circulation of plasmin and thrombin. Characterized by a cycle of intravascular clotting followed by active bleeding because of accelerated consumption of coagulation factors, platelets, and diffuse fibrinolysis.
|
|
|
Predisposing factors for developing an embolus
|
- prethrombotic condition predispose and individual to development of a thrombi. Generally caused by hypercoagulabilty or thrombophillic disorders. Acquired hypercoagulable states are caused by most commonly major surgery (orthopedic), acute MI, CHF, limb paralysis, spinal injury, malignancy, advanced age, post partum period, bedrest greater than 1 week. Also increased with age previous thrombi development and hereditary factors.
|
|
|
schematic drawing circulatory system
|
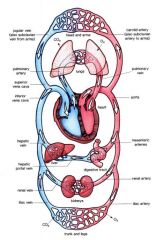
|
|
|
Dyspnea
|
Dyspnea or dyspnoea (pronounced disp-nee-ah, IPA /dɪsp'niə/), from Latin dyspnoea, from Greek dyspnoia from dyspnoos, shortness of breath) or shortness of breath (SOB) is a debilitating symptom that is the experience of unpleasant or uncomfortable respiratory sensations.[1] It is a common symptom of numerous medical disorders, particularly those involving the cardiovascular and respiratory systems; dyspnea on exertion is the most common presenting complaint for people with respiratory impairment
|
|
|
Clubbing
|
is a deformity of the fingers and fingernails that is associated with a number of diseases, mostly of the heart and lungs
|
|
|
Hypoxemia
|
s generally defined as decreased partial pressure of oxygen in blood,[1][2][3][4] sometimes specifically as less than 60 mmHg (8.0 kPa)[3][4] or causing hemoglobin oxygen saturation of less than 90%
|
|
|
Hemoptysis
|
is the expectoration (coughing up) of blood or of blood-stained sputum from the bronchi, larynx, trachea, or lungs (e.g. in tuberculosis or other respiratory infections
|
|
|
Cyanosis
|
is a blue coloration of the skin and mucous membranes due to the presence of > 5g/dl deoxygenated hemoglobin in blood vessels near the skin surface.
|
|
|
Hypoxia
|
s a pathological condition in which the body as a whole (generalized hypoxia) or a region of the body (tissue hypoxia) is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise. A mismatch between oxygen supply and its demand at the cellular level may result in a hypoxic condition. Hypoxia in which there is complete deprivation of oxygen supply is referred to as anoxia
|
|
|
Atelectasis
|
is a medical condition in which the lungs are not fully inflated. It may affect part or all of one lung.[1] It is a condition where the alveoli are deflated, as distinct from pulmonary consolidation.
It is a very common finding in chest x-rays and other radiological studies. It may be caused by normal exhalation or by several medical conditions. Although frequently described as a collapse of lung tissue, atelectasis is not synonymous with a pneumothorax, which is a more specific condition that features atelectasis. Acute atelectasis may occur as a post-operative complication or as a result of surfactant deficiency. In premature neonates, this leads to infant respiratory distress syndrome. |
|
|
Acute Respiratory Failure
|
Acute respiratory distress syndrome is a type of respiratory (lung) failure resulting from many different disorders that cause fluid to accumulate in the lungs and oxygen levels in the blood to be too low.
|
|
|
signs and symptoms of clubbing
|
Clubbing develops in five steps:[3]
1. Fluctuation and softening of the nail bed (increased ballotability) 2. Loss of the normal <165° angle (Lovibond angle) between the nailbed and the fold (cuticula) 3. Increased convexity of the nail fold 4. Thickening of the whole distal (end part of the) finger (resembling a drumstick) 5. Shiny aspect and striation of the nail and skin |
|
|
patho of clubbing
|
The exact cause for sporadic clubbing is unknown, and there are numerous theories as to its cause. Vasodilation (distended blood vessels), secretion of growth factors (such as platelet-derived growth factor and hepatocyte growth factor) from the lungs, and other mechanisms have been proposed. The discovery of disorders in the prostaglandin metabolism in primary osteo-arthropathy has led to suggestions that overproduction of PGE2 by other tissues may be the causative factor for clubbing
|
|
|
Perfusion – p1194-1196
|
The circulation of blood through the tissues. Passing of a fluid through spaces. Supplying of an organ or tissue with nutrients and oxygen by injecting blood or a suitable fluid into an artery.
|
|
|
Discuss how changes in compliance and elastic recoil effect ventilation. p 1191
|
is the tendency of the lungs and chest wall to return to their resting state after inspiration. The elastic recoil forces of the lungs and chest wall are in opposition and pull on each other, creating the normally negative pressure of the pleural space.
|
|
|
Describe the role of the nervous system p 1188
|
Ventilation is controlled by the sympathetic and parasympathetic division of the utonomic nervous system, which adjust airway caliber by causing bronchial smooth muscle to contract or relax and control the rate and depth of ventilation. Neuroreceptors in the lungs (lung receptors) monitor the mechanical aspects of ventilation. Irritant receptors sense the need to expel unwanted substances, stretch receptors sense lung volume (lung expansion), and j-receptors sense alveolar size.
|
|
|
The role of chemicals in regulating ventilation p 1188
|
Chemoreceptors in the circulatory system and brain stem sense the effectiveness of ventilation by monitoring the pH status of verebrospinal fluid and oxygen content (pO2) of arterial blood.
|
|
|
Discuss the changes that take place in the respiratory system during the aging process p1201
|
affects the mechanical aspects of ventilation by decreasing chest wall compliance and elastic recoil of the lungs. Changes in these elastic properties reduce ventilator reserve. Aging causes the Pa02, to decrease but does not affect the PaCO2.
|
|
|
Describe the pressures in the respiratory system that maintain its effectiveness and relate what does happen if there is a change in pulmonary pressures
|
Oxygen is loaded onto hemoglobin by the driving pressure excerted by Pao2, in the plasma. As pressure decreases at tissue level, oxygen dissociates from hemoglobin and enters tissue cells by diffusion, again down the concentration gradient.
|
|
|
Provide two specific causes of atelectasis p1212, 1259
|
is the collapse of alveoli resulting from compression of the lung tissue or absorption of gas from obstructed alveoli.
|
|
|
Discuss the effects of a break in visceral pleura p1202
|
The pleura that covers the lungs and enters into and lines the interlobar fissures. It is loose at the base and at the sterna and vertebral borders to allow for lung expansion.
|
|
|
Pneumothorax p1213
|
air or gas in the pleural space. Simple or communicating pneumothorax - The pressure of the pleural space is the same as atmospheric pressure. There can be either a hole in the chest wall that disrupts the pleura or a hole in the lung (breakage of a bleb causing a spontaneous pneumothorax). Air moves into the chest cavity with inspiration and out with expiration. Tension pneumothorax - Air enters the chest cavity on inspiration, but sometimes there is a flap of skin or pleura or lung that acts as a one- way valve. This valve then closes on exhalation and there is retention of inspired air in the chest cavity. As the amount of air increases, pressure is exerted on the already collapsed lung as well as the heart, trachea, and great vessels displacing these organs towards the unaffected side. This is a life threatening condition. Clinical manifestations of pneumothorax: Tracheal shift to opposite side, Absent breath sounds with hyperresonance, X-ray shows a dark area with mediastinal shift, Hypoxemia and respiratory distress, Subcutaneous emphysema. Treatment: Insertion of an irritant that causes inflammation and scarring of the pleura. This is done if the pleura does not heal on its own, Chest tube placement connected to a water-seal drainage system until the pleura is healed.
|
|
|
flail chest p1217
|
Flair chest results from rib or sterna fractures that disrupt the mechanics of breathing.
|
|
|
Map out the cellular events that contribute to adult respiratory distress syndrome p1269
|
Etiology - any disease or condition that leads to a decrease in blood flow can cause ARDS. your text and your workbook have good schematic drawings of the pathogenesis of ARDS (see Fig. 33-8 on page 1219 in textbook) so the pathogenesis of ARDS will not be discussed here. I do want to note that the chemical mediators of the inflammatory response also attack other membranes, especially the kidney. The basement membrane of the lung and the basement membrane of the kidney are the same, so there is usually kidney damage. There may indeed be multi systems failure, or as your book calls it, multiple organ dysfunction syndrome. The onset of ARDS after the initial injury can happen immediately or over hours.
|
|
|
Compare and contrast the diseases that compromise the term "chronic obstructive pulmonary disease" 1. Chronic bronchitis p1224
|
causes airway obstruction resulting from bronchial smooth muscle hypertrophy and production of thick, tenacious mucus.
|
|
|
Compare and contrast the diseases that compromise the term "chronic obstructive pulmonary disease" 1. emphysema p1224
|
destruction of the alveolar septa and loss of passice elastic recoil lead to airway collapse and obstruct gas flow during expiration. Septal deterioration is caused by antitrypsin deficiency or old age tends to be panacinar. Caused by smoking.
|
|
|
Describe the pathogenesis of Tuberculosis p1230
|
caused by mycobacterium tuberculosis. The inflammatory response isolates colonies of bacilli by enclosing them in tubercles and surrounding the tubercles with scar tissue. Bacilli may remain dormant within the tubercles for life or, if the immune system breaks down, cause recurrence of active disease.
|
|
|
clinical manifestations of Tuberculosis p1230
|
fatigue, weight loss, lethargy, anorexia, low grade fever. Cough that produces purulent sputum develops slowly and becomes more frequent over several weeks or months. Night sweats and general anxiety. Dyspnea, chest pain, hemoptysis. TB and HIV produce neurological deficits, meningitis symptoms, bone pain and urinary symptoms.
|
|
|
Discuss the causes of pulmonary embolus p1232
|
Pulmonary embolism is occlusion of a portion of the pulmonary vascular bed by a thrombus, a tissue fragment, or an air bubble. Depending on its size and location, the embolus can cause hypoxic vasoconstriction, pulmonary edema, atelectasis, pulmonary hypertension, shock, and even death. Most pulmonary emboli originate from a blood clot in the legs or pelvis. These clots are usually the result of immobility, oral contraceptives, obesity, pregnancy, blood stasis, and vessel damage. The blood clots can also be caused by endocarditis, cardioversion of atrial fibrillation, and hypercoagulability from polycythemia.
|
|
|
cellular changes in pulmonary embolus p1232
|
??
|
|
|
clinical manifestations of pulmonary embolus p1232
|
Moderate emboli causes sudden dyspnea, pleuritic pain, friction rub, pulmonary hypertension, a normal chest x-ray but decreased perfusion to the area on a lung scan. Massive emboli/infarction features the above plus shock, cyanosis, right ventricular failure, hemoptysis, engorged neck veins, and death (30% to 50% mortality).
|
|
|
treatments, outcomes pulmonary embolus 1232
|
Bed rest to decrease oxygen consumption, Drugs to dissolve the clot (thrombolytics) and anticoagulants (heparin), Oxygen, Ventilator support., An umbrella in the descending vena cava for those who have chronic showers of emboli. The umbrella keeps the clots from reaching the lungs, The best "treatment" is prevention. A risk assessment can determine who is apt to develop deep leg thrombosis. Early ambulation postoperatively reduces the risk of venous stasis, as does pneumatic calf decompression, and elastic stockings. Many post orthopedic surgery patients (total knees and total hips for example) are put on anticoagulant therapy until they have resumed weight bearing.
|
|
|
complications of pulmonary embolus
|
Massive emboli/infarction features the above plus shock, cyanosis, right ventricular failure, hemoptysis, engorged neck veins, and death (30% to 50% mortality).
|
|
|
Describe the various cancers of the pulmonary system paying special attention to the causes – p1236
|
Lip cancer, laryngeal cancer, lung cancer, bronchial carcinoid and adenocystic tumors of the bronchial airways.
|
|
|
the causes of the various cancers of the pulmonary system p1238
|
cigarette smoking, passive smoking, occupational risk factors including asbestos, dust, arsenic, chromium, nickel, ionizing radiation, coal, mustard gas, vinyl chloride.
|
|
|
the various cancers of the pulmonary system clinical manifestations p1238
|
persistant cough, sputum streaked with blood, chest pain, recurring attacks of pneumonia or bronchitis.
|
|
|
treatment options of the various cancers of the pulmonary system p1238
|
surgery, radiation, chemo. Surgery is the treatment of choice.
|
|
|
prognosis of the various cancers of the pulmonary system p1238
|
depends on the cancer. Lung cancer 15% of white, 12% blacks live 5 or more years.
|
|
|
State the function of each circuit of the circulatory system pg 1029-1033
|
In the systemic circuit, blood leaves the heart through the aorta, goes to all the organs of the body through the systemic arteries, and then returns to the heart through the systemic veins. Thus there are two circuits. Arteries always carry blood away from the heart and veins always carry blood toward the heart. Most of the time, arteries carry oxygenated blood and veins carry deoxygenated blood. There are exceptions. The pulmonary arteries leaving the right ventricle for the lungs carry deoxygenated blood and the pulmonary veins carry oxygenated blood. If you are confused as to which way the blood flows through the heart, try this saying "When it leaves the right, it comes right back, but when it leaves the left, it's left." The blood does not have to travel as far when going from the heart to the lungs as it does from the heart to the toes. It makes sense that the heart would be larger on one side than on the other. When you look at a heart, you see that the right side of the heart is distinctly smaller than the left side, and the left ventricle is the largest of the four chambers.
|
|
|
Describe cardiac action potential relating it to the conduction system of the heart and ECG tracings. pg. 1037
|
The cardiac action potential is a specialized action potential in the heart, with unique properties necessary for function of the electrical conduction system of the heart.
As an electrical impulse passes from cell to cell (fiber to fiber) in the myocardium, it stimulates the fibers to shorten. shortening causes muscular contraction, or systole. after the action potential passes, the fibers relax and return to their resting length, causing diastole. The muscle fibers of the myocardium are uniquely joined so that action potentials pass from cell to cell very rapidly and efficiently. therefore an action potential generated in on part of the myocardium passes almost simultaneously through all its contiguous fibers, causing rapid contraction. |
|
|
Frank-Starling Law pg 1047
|
states that the myocardial fiber stretch determines the force of myocardial contraction (the greater the stretch, the stronger the contraction).
|
|
|
Laplace’s Law
|
states that the amount of contractile force generated within a chamber depends on the radius of the chamber and the thickness of its wall (the smaller the radius and the thicker the wall, the greater the force of contraction).
|
|
|
Right Coronary artery supplies- pg 1035
|
R. Atrium
R. Ventricle Sinus Node AV node Bundle of His |
|
|
Left Coronary Artery - Anterior Descending pg 1035
|
Anterior L. Ventricle
Apex Intraventricular septum R. Bundle L. Anterior bundle L. Posterior bundle |
|
|
Left Coronary Artery - Circumflex pg 1035
|
L. Atrium
Posterior L. Ventricle Anterior L. Ventricle (with LAD) Lateral wall of L. Ventricle |
|
|
Identify the anatomical structures that take part in gas exchange. P 1181
|
Lungs, airways, chest wall, and pulmonary and bronchial circulation.
|
|
|
Conducting airways -1181
|
nasopharynx, oropharynx, trachea, bronchi, and bronchioles to the sixteenth division
|
|
|
Acinus or gas exchange airways- pg 1185
|
respiratory bronchioles, alveolar ducts, and alveoli
|
|
|
Respiratory bronchioles-1185
|
- the bronchioles from the 16-23rd contain increasing #’s of alveoli
|
|
|
Aveoli- 1185-
|
are the primary gas-exchange units of the lung, where o2 enters the blood and co2 is removed
|
|
|
Surfactant- 1185-
|
a lipoprotein that coats the inner surface of the alveolus and facilitates its expansion during inspiration.
|
|
|
Successful venitilation-1188
|
involves the mechanics of breathing, the interactions of forces and counterforces involving the muscles of inspiration and expiration, alveolar surface tension, elastic properties of the lungs and chest wall, and resistance to airflow.
|
|
|
Anatomic dead space
|
is the space in the conducting airways where no gas exchange takes place from nose to the acinus (respiratory bronchioles, alveolar ducts and alveoli).
|
|
|
Physiologic dead space
|
is the volume of inspired gas that does not take part in gas exchange. There are healthy alveoli that have filled with inspired air, but there is no blood supply (perfusion) to the alveoli so there is no gas exchange. The measure of dead space incorporates anatomic dead space + physiologic dead space. Normally, the pressure of oxygen in the alveoli should equal the oxygen pressure of arterial oxygen.
|
|
|
Distribution of pulmonary ventilation p 1192
|
less negative pressure at the apex of the lung, so the alveoli at the top of the lung remain more distended and fill less with respiration. As you progress down the lung, there is more negative pressure at the bases so they are less distended. The greatest ventilation is to the dependent lung fields. If there is unequal resistance due to obstruction or bronchial constriction, the greatest amount of ventilation occurs at the area of least resistance.
|
|
|
diffusion p1196
|
-is a passive process Rate of diffusion depends on surface area, thickness of alveolar/capillary membrane, and concentration gradient. CO2 diffuses 20 times faster than oxygen and is 25 times more soluble
|
|
|
five layers the gases must go through in diffusion pg 1196
|
1. Red blood cell
2. Capillary membrane (1 cell thickness) 3. Interstitial fluid 4. Alveolar membrane 5. Surfactant |
|
|
State understanding of the concept of ventilation/perfusion mismatch p1196
|
Matching is the goal. Ideally, all alveoli are perfused (have blood flowing to them) and aerated so gas exchange can take place. There is some normal mismatching, but it is minimal.
|
|
|
Poor ventilation with adequate perfusion p1196
|
The most common causes of this mismatch are atelectasis (collapsed alveoli) and splinting of the chest wall. We splint when taking a deep breath causes pain, so we don't breath with part of the chest thus air does not get to the alveoli in that area. When this happens, there is mixing of oxygenated blood and unoxygenated blood (venous admixture or a shunt). This venous admixture then goes to the systemic circulation.
|
|
|
Adequate ventilation with poor perfusion. Pg 1196
|
The most common cause of the mismatch is a pulmonary emboli which blocks blood supply to an area of the lung. Ventilation is being wasted and there is added dead space.
|
|
|
"right to left shunt" pg 1196
|
as with poor ventilation and adequate perfusion there is still some air in the alveoli with ventilation, so there is some gas exchange. There are times however, when there is a vessel that is carrying unoxygenated blood that totally bypasses all alveoli. This unoxygenated blood is added to arterialized blood coming from ventilated alveoli. This is called a "true shunt" or a right-to-left shunt. So, when you come upon that term, just realize that unoxygenated blood is being mixed with oxygenated blood decreasing the amount of oxygen available to the tissues
|
|
|
Discuss how changes in compliance and elastic recoil effect ventilation. p 1191
|
is the tendency of the lungs and chest wall to return to their resting state after inspiration. The elastic recoil forces of the lungs and chest wall are in opposition and pull on each other, creating the normally negative pressure of the pleural space
|
|
|
Describe the oxyhemoglobin dissociation curve and its importance in assessing effective gas exchange p1198
|
Oxygen forms a molecular bond with the iron molecule of the Hb protein. The Hb protein can change in conformation. This conformational change alters the affinity of the iron for oxygen. When looking at the Oxyhemoglobin Dissociation Curve, you will notice that the amount of oxygen carried by hemoglobin (saturation) doesn't change much if the PaO2 is 60 mmHg or above. Once the partial pressure of oxygen gets to just below 60, there is a rapid decrease in saturation. Shift to the right means the Hb has less affinity for O2. Hb lets the oxygen off to the tissues more quickly. This can be a good thing for a while as the tissues, when in state of acidosis, can use the oxygen. Shift to the left means the Hb has a high affinity for oxygen. The Hb holds on to the oxygen, not releasing it for use by the tissues.
|
|
|
Influence of ANS on cardiac activity- pg 1058
|
Major control center in the medulla of the brain stem which regulates hr and bp. Neurons from medulla connect with Parasympathetic nervous system usually depresses cardiac function is usually in control of the heart rate at rest. Sympathetic nervous system inhibits the parasympathetic nervous system. Hypothalamus - regulates cardiac response to temperature changes Cerebral Cortex - adjusts cardiac reaction to emotional states
|
|
|
Influence of barorectoptors and chempreceptors on cardiac activity 1058
|
Baroreceptors (pressoreceptors) on the aortic and carotid arteries sense changes in blood pressure and with increased pressure, initiate a parasympathetic response and with decreased pressure, initiate a sympathetic response.
|
|
|
Three layers of blood vessels- pg 1053
|
tunica intima (innermost or intimal layer) tunica media (middle or medial layer) and tunica externa (outermost or medial layer).
|
|
|
Observable signs of cv disease- 1069
|
decreased loc (caused by insufficient perfusion of brain tissue) and cyanosis particularly of the mucus membrane
|
|
|
Palpable signs of cv disease-1069
|
abnormal pulses of the radial, femoral and carotid arteries
|
|
|
Auditory signs of cv diesease-1069
|
- abnormal heart sounds detected by auscultation by stethoscope or phonocardiography.
|
|
|
Stress tests 1072
|
clinical manifestations of cv disease that might not be present at rest.
|

