![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
105 Cards in this Set
- Front
- Back
|
What do you need to do when a patient comes in with an altered mental status?
|
- Consider a broad differential diagnosis (systemic diagnostic approach)
- Do a neurological exam (look for subtle deficits) - Look for quickly reversible causes before broad work-up - "DON'T" forget: coma cocktail - dextrose, oxygen, naloxone, thiamine |
|
|
What should you give to a patient in a coma that may reverse it?
|
DON'T forget:
- Dextrose - Oxygen - Naloxone - Thiamine |
|
|
How common are patients with altered mental status presenting to the ED?
|
3% of the patients in ED are altered
|
|
|
What are the causes of altered mental status in patients presenting to ED?
|
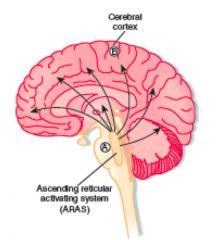
- 85% metabolic or systemic derangements
- 15% structural lesions (eg, reticular activating system - bleed, infarct, tumor, etc) |
|
|
What are the components of consciousness?
|
- Arousal
- Cognition |
|
|
What is arousal? Structural contribution?
|
Awareness of self and surroundings
- Ascending reticular activating system - Controls input of somatic and sensory stimuli, arousal from sleep - Vulnerable to small lesions in brainstem (eg, bleeds, infarcts, tumors) |
|
|
What is cognition? Structural contribution?
|
Combination of orientation, judgment, and memory
- Cerebral cortex: unilateral lesions rarely cause altered mental status, but possibly a bilateral lesion |
|
|
What is the definition of a coma?
|
Unconsciousness for >6 hours
- Cannot be awakened - No response to painful stimuli, light, or sound - No normal sleep-wake cycle - No voluntary actions |
|
|
What can cause a coma?
|
– Damage to brainstem, cortex, both
– Susceptible to toxins, metabolic derangements, mechanical injury |
|
|
What are the causes of a coma more common in infants?
|
- Infection
- Trauma, abuse - Metabolic |
|
|
What are the causes of a coma more common in children?
|
Toxic ingestion
|
|
|
What are the causes of a coma more common in adolescents or young adults?
|
- Toxic ingestion
- Recreational drug use - Trauma |
|
|
What are the causes of a coma more common in elderly?
|
- Med changes
- OTC meds - Infection - Alterations in living environment - Stroke - Trauma |
|
|
What are the structural causes of altered mental status and coma?
|
- Trauma
- Stroke syndromes (embolism or thrombosis) - Hemorrhage - Tumor - Pituitary apoplexy - Acute hydrocephalus - Infection |
|
|
What do you need to always evaluate in a patient?
|
ABCs
- Airway - Breathing - Circulation |
|
|
What is AMS?
|
Altered Mental Status
|
|
|
What is LOC?
|
Loss of Consciousness and Level of Consciousness
|
|
|
What are the sources of information for a patient with AMS?
|
- EMS (emergency medical services)
- Family - EMR (electronic medical record) |
|
|
What is the the scoring system used for patients with AMS?
|
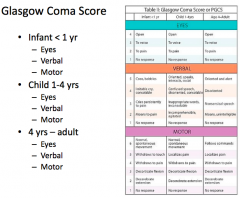
Glasgow Coma Scale (GCS)
- Eyes = out of 4 - Verbal = out of 5 - Motor = out of 6 |
|
|
What is the best "eye response" on the GCS?
|
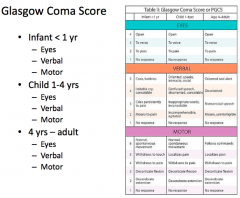
Eye opening = 4
|
|
|
What is the best "verbal response" on the GCS?
|
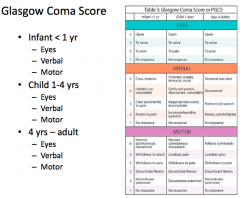
Oriented and alert / speaking / social = 5
|
|
|
What is the best "motor response" on the GCS?
|
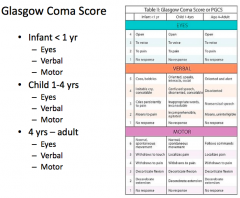
- Normal, spontaneous movement (in infants/children)
- Follows commands (adults) = 6 |
|
|
What is the worst "eye response" on the GCS?
|
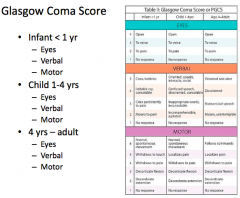
No response - no opening, pain, verbal command, spontaneous = 1
|
|
|
What is the worst "verbal response" on the GCS?
|
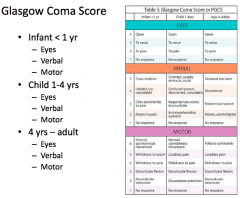
No verbal response - incomprehensible sounds, inappropriate words, confused, oriented = 1
|
|
|
What is the worst "motor response" on the GCS?
|
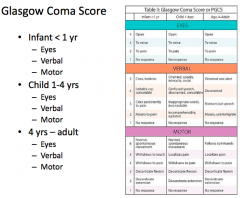
No motor response, extends to pain, flexes to pain, withdraw from pain, localize to pain, obeys commands = 1
|
|
|
How do you work up a patient with altered mental status?
|
- Basic neuro exam
- Differential - "DON'T" coma cocktail - Labs: electrolytes, etc - Imaging: head CT, etc - Others |
|
|
What sorts of things can treat patients with altered mental status?
|
- Antidotes
- Antibiotics - Surgery - Supportive care - Metabolic cofactors |
|
|
Consciousness is best described by which of the following?
a) Level of arousal b) Level of cognition c) Level of response to pain d) Level of arousal and cognition e) Level of arousal and response to pain |
Level of arousal and cognition
|
|
|
Case 1:
- 1 day old M born at term, still in hospital, called to bedside because he is lethargic - ABCs: Airway- intact, Breathing- slow respirations, Circulation- femoral pulses intact, capillary refill 3 seconds (≤3 sec is not good) - C-section due to size, poor suck, +prenatal care - PMH: mother T1DM (insulin-dependent) What are our thoughts of what could be causing the problems in this baby? What should we check? |
* Hypoglycemia d/t exogenous insulin in mom (check his glucose - it is in fact low, 20)
- Also check for infection/sepsis (eg, group B strep) - Maybe he has an inborn error of metabolism - Shaken baby syndrome (trauma) |
|
|
What would your differential diagnosis be if the child was 6 weeks old, had no prenatal care, and was febrile to 38°C, and came in lethargic?
|
- Infection
- Honey - contains botulinum toxin - Trauma |
|
|
Case 1:
What would your differential diagnosis be if this child were 1 year old, hypoglycemic, lethargic, just at grandma’s house, who has PMH: DM and HTN? |
Ingestion of these can all cause hypoglycemia:
- Sulfonylurea (eg, glipizide) - β-blockers - Alcohol |
|
|
What kind of symptoms occur in a patient with hypoglycemia?
|
Central nervous system (CNS) symptoms predominate
– Brain relies almost entirely on glucose – During prolonged starvation, the brain can use ketones – Other major organs (heart, liver, and skeletal muscle) function during hypoglycemia (use various fuel sources (ie., fatty acids)) |
|
|
How does the density of neuronal insulin receptors vary in diabetics?
|
Varies depending on glycemic control
– Poor glycemic control → fewer neuronal glucose receptors • Hypoglycemic symptoms at higher concentrations of glucose – Mean [glucose] for symptomatic hypoglycemia 78 ± 5 mg/dL versus 53 ± mg/dL |
|
|
What is the mechanism of Sulfonylureas?
|
- Normally, insulin released with elevation of intracellular ATP
- Sulfonylureas potentiate the effects of ATP at its "sensor" – Increased intracellular K+ → increased intracellular potential opens voltage-gated Ca2+ channels → increases intracellular calcium concentration – Increased calcium → release of insulin – Binding of sulfonylureas to receptor sites → insulin release |
|
|
What is the mechanism of Meglitinides?
|
- Bind to K+ channels on pancreatic cells → increased insulin secretion
- Hypoglycemic effects shorter duration |
|
|
What are the effects of Metformin?
|
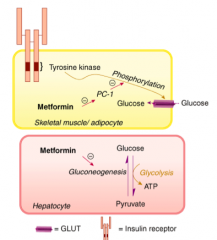
Glucose-stabilizing by several mechanisms
– Inhibits gluconeogenesis → decreased hepatic glucose output – Enhanced peripheral glucose uptake – Decreased fatty acid oxidation – Increased intestinal use of glucose – In skeletal muscle and adipose cells, enhanced activity and translocation of glucose transporters |
|
|
What is the mechanism of Metformin?
|
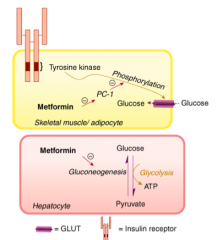
- Under normal conditions, insulin binding to its receptor on myocytes and adipocytes activates tyrosine kinase, resulting in phosphorylation and activation of the membrane-bound glucose transporter GLUT.
- Non-insulin-dependent diabetes mellitus is causally associated with an increased activity of PC-1, a glycoprotein that inhibits tyrosine kinase activity and thus reduces myocyte and adipocyte glucose uptake. - Metformin reduces PC-1 activity in these cells, enhancing peripheral glucose utilization. - In addition, gluconeogenesis in hepatic cells is reduced through interference with pyruvate carboxylase, the enzyme responsible for conversion of pyruvate to oxaloacetate. |
|
|
Which of the following medications would not cause hypoglycemia?
a) Sulfonylurea b) Beta-blocker c) Insulin d) Metformin |
Metformin
|
|
|
Case 2:
- 17 yo F presents with altered mental status, she was found on doorstep teasing her at at 0200 by parents - ABCs: Airway- intact, Breathing- normal breath sounds bilaterally, Circulation- bounding peripheral pulses, tachycardia - She had been at a party with some friends, possibly used drugs, no PMH What is the differential diagnosis? |
- Drugs with alcohol (not alcohol on its own)
- Infection - Withdrawal (hopefully not in a 17 yo) - Traumatic brain injury (eg, brain bleed) |
|
|
Case 2:
- 17 yo F presents with altered mental status, she was found on doorstep teasing her at at 0200 by parents - ABCs: Airway- intact, Breathing- normal breath sounds bilaterally, Circulation- bounding peripheral pulses, tachycardia - She had been at a party with some friends, possibly used drugs, no PMH How would the differential diagnosis be different if she was a known alcoholic? |
Alcohol withdrawal
|
|
|
Which drugs stimulate the SNS? Types?
|
Sympathomimetics
- Direct-acting: bind post-synaptic receptors → direct stimulation - Indirect-acting: increase synaptic concentration of NTs |
|
|
Which sympathomimetics should we be familiar with?
|
- Dobutamine (direct acting β-agonist)
- Amphetamine (indirect acting) - Cocaine (indirect acting) - Bupropion (inhibits NE uptake) - Venlafaxine (inhibits NE uptake) |
|
|
How do direct-acting sympathomimetics act?
|
Bind post-synaptic receptors → direct stimulation
|
|
|
How do indirect-acting sympathomimetics act?
|
Increase synaptic concentration of NTs
- May block NT uptake - May stimulate NT release |
|
|
Which of the following sympathomimetic drugs does not inhibit the uptake of norepinephrine?
a) Cocaine b) Amphetamine c) Venlafaxine d) Bupropion e) Dobutamine |
Dobutamine (direct-acting)
|
|
|
What are the symptoms of patients with sympathomimetic overdose / toxidrome?
|
• Hypertension
• Hyperthermia • Tachycardia • Mydriasis • Diaphoresis • Agitation |
|
|
What is a toxidrome?
|
Describes groups of signs and symptoms consistently attributed to particular toxins
– Usually a combination of vital signs and end organ damage (clinically apparent) |
|
|
What are the symptoms in an anti-cholinergic toxidrome?
|

|
|
|
What are the symptoms in a cholinergic toxidrome?
|

|
|
|
What are the symptoms in an opioid toxidrome?
|

|
|
|
What are the symptoms in a sympathomimetic toxidrome?
|

|
|
|
What are the symptoms in a sedative hypnotic toxidrome?
|

|
|
|
What is the constellation of symptoms you would expect to see with sympathomimetic intoxication?
a) Tachycardia b) Elevated temperature c) Mydriasis d) A and B only e) All of the above |
All of the above
|
|
|
If you were to give medications to calm this patient (or if you were performing procedural sedation) – what would you give?
|
You would give benzos, then flumazenil, unless they were prescribed this at home, to avoid withdrawal
|
|
|
What is the mechanism of Flumazenil?
|
Competitive benzo receptor antagonist
|
|
|
What are the uses / effects of Flumazenil?
|
• Role in patients with unknown overdose is limited because seizures and dysrhythmias may develop when the effects of a benzodiazepine are reversed in a mixed overdose.
• Can induce benzodiazepine withdrawal symptoms, including seizures in patients who are benzodiazepine dependent. |
|
|
When can you give Flumazenil to treat overdoses?
|
- Patients who are naive to benzos and who overdose solely on a benzo
- Patients who are naive to benzos whose benzo component must be reversed after procedural sedation |
|
|
Case 3:
- 50 yo male found down by family in bathroom - ABCs: Airway- intact, Breathing- RR 4, Circulation- weak pulses and cyanotic - Hx: chronic pain, pain contract, multiple ED visits for pain What is the differential diagnosis? |
- Intoxication (EtOH, benzo, opioid)
- Cardiac arrest - Hypoglycemia - Hyperglycemia - Traumatic Brain Injury (TBI) - Sepsis - Meningitis |
|
|
What are the components of the Opioid Toxidrome?
|
- Altered mental status (stupor, coma)
- Miosis (constricted pulses) - Respiratory depression |
|
|
What do you give to a patient who has overdosed on opioids?
|
Naloxone (titrate up in dosing as they can go crazy when they "wake up")
|
|
|
What is the action of Opioids?
|
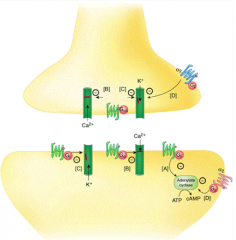
- Activates G-protein on binding - reduces capacity of AC to make cAMP
- Closes Ca2+ channels - reduces signal to release NTs - Open K+ channels - hyperpolarizes cells, decreasing cell activity) |
|
|
What are the conventional names for the opioid receptors?
|
- μ1
- μ2 - κ1 - κ2 - κ3 - δ - Nociceptin / orphanin FQ |
|
|
What are the important clinical effects of μ1 receptor agonists?
|
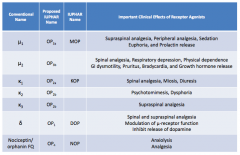
- Supraspinal analgesia
- Peripheral analgesia - Sedation - Euphoria - Prolactin release |
|
|
What are the important clinical effects of μ2 receptor agonists?
|
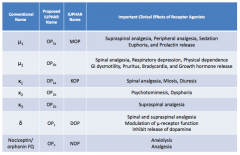
- Spinal analgesia
- Respiratory depression - Physical dependence - GI dysmotility - Pruritus - Bradycardia - GH release |
|
|
What are the important clinical effects of κ1 receptor agonists?
|
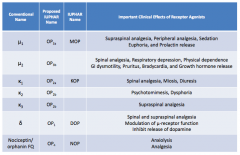
- Spinal analgesia
- Miosis - Diuresis |
|
|
What are the important clinical effects of κ2 receptor agonists?
|
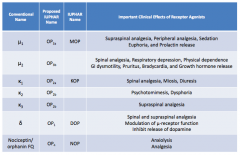
- Psychomimesis
- Dysphoria |
|
|
What are the important clinical effects of κ3 receptor agonists?
|
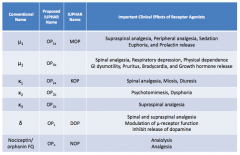
Supraspinal analgesia
|
|
|
What are the important clinical effects of δ receptor agonists?
|
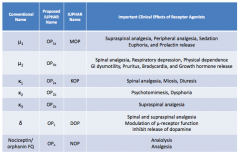
- Spinal and supraspinal analgesia
- Modulation of μ-receptor function - Inhibits release of dopamine |
|
|
What are the important clinical effects of nociceptin / orphanin FQ receptor agonists?
|
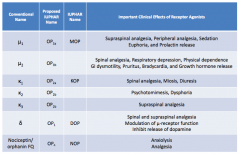
- Anxiolysis
- Analgesia |
|
|
What are the effects of opioids?
|
- Analgesia
- Cough suppression - Anti-diarrheal effect / constipation - Euphoria - Sedation - Respiratory depression - Nausea - Endocrine effects - Pupillary constriction (miosis) |
|
|
What are the analgesic effects of opioids? Exceptions?
|
- Most full agonists are equally effective except for codeine and propoxyphene
- Decrease both perception and appreciation of pain |
|
|
What are the cough effects of opioids? Types?
|
Cough suppression
- Codeine and Dextromethoprhan - Acts at brainstem cough centers - may not be mediated by opioid receptors |
|
|
What are the GI effects of opioids? Mechanism?
|
Anti-diarrheal effect and constipation
- Central and peripheral effects - Acts on Mu (μ) receptors on GI nerves - Increased anal sphincter tone - Reduced motility Nausea - Stimulation of CTZ in area postrema - More when standing Increased biliary tract pressure Reduced gastric acid secretion |
|
|
What are the other effects of opioids?
|
- Euphoria: rush and high
- Sedation |
|
|
What are the pulmonary effects of opioids? Mechanism?
|
Respiratory Depression
- Most serious side effect - Decreased sensitivity of brainstem chemoreceptors to CO2 - Acute lung injury - Bronchospasm (histamine) |
|
|
What is the most serious side effect of opioids?
|
Respiratory Depression
- Decreased sensitivity of brainstem chemoreceptors to CO2 (which is the most important stimulus to breathing, greater than a lack of O2) |
|
|
What are the endocrine effects of opioids? Mechanism?
|
- Decreased LH release (via μ)
- Increased ADH secretion (via μ) - Decreased ADH secretion (via κ) - Increased Prolactin release - Decreased Gonadotropin release |
|
|
What are the effects of opioids on pupils? Mechanism?
|
Miosis (constricted pupil)
- Stimulates Edinger-Westphal nucleus of oculomotor nerve - No tolerance to this effect |
|
|
What are the cardiac effects of opioids?
|
- Orthostatic hypotension
- Bradycardia - Peripheral vasodilation |
|
|
What are the dermatologic effects of opioids?
|
- Itching
- Flushing (via histamine release) |
|
|
What are the neurologic effects of opioids?
|
- Analgesia
- Anti-tussive (cough suppressant) - Euphoria - Sedation - Coma - Seizures (meperidine, propoxyphene) |
|
|
What are the opioid agonists?
|
- Heroin
- Codeine and Oxycodone - Meperidine - Morphine - Methadone - Fentanyl |
|
|
What are the opioid antagonists?
|
- Naloxone
- Naltrexone |
|
|
Which opioid agonist is not effective on severe pain?
|
Codeine (morphine like efficacy is not achievable at any dose)
|
|
|
How is Codeine typically given?
|
In combination with aspirin or acetaminophen
|
|
|
What happens to heroin in the body?
|
Converted to 6-mono-acetyl morphine and morphine
|
|
|
What is the strongest opioid agonist?
|
Fentanyl - 100x as potent as morphine
|
|
|
Which opioid agonist can be used in treatment of opioid abuse and chronic pain?
|
Methadone
|
|
|
Which opioid agonist forms a toxic metabolite?
|
Meperidine
- Forms toxic metabolite, normeperidine that can accumulate with frequent use - Also interacts with MAO-Is |
|
|
What are the two opioid antagonists? Which has a longer duration of action?
|
- Naloxone - 1-2 hours of duration
- Naltrexone - 24+ hours of duration |
|
|
Which drug is combined with opioids to decrease parenteral abuse liability?
|
Naloxone
|
|
|
Which drug is used in treatment of alcoholism and opiate addiction?
|
Naltrexone
|
|
|
Which drug is a component of newer treatments for addiction?
|
Suboxone / Naloxone
|
|
|
In a patient with altered mental status who has a history of alcoholism, what should you consider? Types?
|
Thiamine deficiency:
- Wet beriberi / CV disease - Dry beriberi / Wernicke-Korsakoff Syndrome |
|
|
What are the signs of "wet" beriberi?
|
CV disease
- High-output cardiac failure - Peripheral vasodilation - Formation of AV fistulae |
|
|
What are the signs of "dry" beriberi?
|
Wernicke-Korsakoff Syndrome
- Classic triad: oculomotor abnormalities, ataxia, and global confusion - 10-20% mortality |
|
|
How do you treat alcoholic or malnourished patients?
|
Thiamine supplementation (vitamin B1) empirically
|
|
|
What are the characteristics of Thiamine?
|
Vitamin B1
- Water-soluble - Essential cofactor in carbohydrate metabolism |
|
|
Case 3:
- 50 yo male found down by family in bathroom - ABCs: Airway- intact, Breathing- RR 4, Circulation- weak pulses and cyanotic - Hx: chronic pain, pain contract, multiple ED visits for pain Which of the following would you use to reverse this patient’s toxidrome? a) Bicarbonate b) Flumazenil c) Glucose d) Naloxone e) Thiamine |
Naloxone
|
|
|
Case 4:
- 50 yo male found down by family in bathroom - ABCs: Airway- intact, Breathing- RR 4, Circulation- weak pulses and cyanotic - Hx: chronic pain, pain contract, multiple ED visits for pain In the patient’s past medical history, he is a recovering alcoholic. At a prior Emergency Department visit several years ago, the patient had presented with Wernicke’s Encephalopathy. Which of the following symptoms is NOT part of this classic triad? a) Agitation b) Confusion c) Ophthalmoplegia d) Ataxia |
Agitation
|
|
|
Case 4:
- 84 yo F found to be altered at assisted living facility - ABCs: Airway- intact, Breathing- RR 12, Circulation- weak central pulses, decreased capillary refill, peripheral cyanosis - Hx: HTN, CAD, DM, COPD - Temp: 38 (elevated); hypotensive What is the differential diagnosis? |
- Sepsis
- Hypothyroidism - Hypoglycemia - Intoxication (EtOH, benzo, opioid) - Cardiac arrest - PE - CVA |
|
|
What is SIRS?
|
Systemic Inflammatory Response Syndrome
- Inflammatory state affecting entire body |
|
|
What are the criteria for SIRS (Systemic Inflammatory Response Syndrome)?
|
Do not need all of these
- Temp >38°C - HR >90 - RR >20 or PaCO2 <32 mmHg - WBC >12,000/mm3, <4000/mm3, or >10% bands |
|
|
What is sepsis?
|
Presence (probably or documented) of infection together with systemic manifestations of infection
- Temp >38°C - HR >90 - RR >20 or PaCO2 <32 mmHg - WBC >12,000/mm3, <4000/mm3, or >10% bands |
|
|
What is severe sepsis?
|
Sepsis + sepsis-induced organ dysfunction or tissue hypoperfusion
|

