![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
State with definite volume and shape is....
|
solid
|
|
|
Three ordinary states of matter are....
|
solid, liquid, gas
|
|
|
State with definite volume and indefinite shape is...
|
liquid
|
|
|
State with indefinite volume and indefinite shape is....
|
gas
|
|
|
State that is easily compressible is...
|
gas
|
|
|
State that has particles rigidly fixed together is....
|
solid
|
|
|
State that has particles touching but able to move past one another is...
|
liquid
|
|
|
State that has the least "glue" holding it together such that the particles move freely about is...
|
gas
|
|
|
What happens to temperature while a state is undergoing a phase change?
|
temp stays the same
|
|
|
When a solid turns into a liquid, we call it...
|
melting
|
|
|
When a liquid turns into a gas, we call it...
|
boiling
|
|
|
When a gas turns back into a liquid, we call it...
|
condensation
|
|
|
When a solid turns directly into a gas, we call it....
|
sublimation
|
|
|
When a gas turns directly into a solid, we call it....
|
deposition
|
|
|
When a liquid turns into a solid, we call it....
|
freezing
|
|
|
All states of matter will e________ when heated.
|
expand
|
|
|
A _____ completely fills its container.
|
gas
|
|
|
A _____ fills its container from the bottom up.
|
liquid
|
|
|
Water freezes at 0 C and boils at 100 C. What state is water at 35 C?
|
Liquid
|
|
|
Water melts at 0 C and boils at 100 C. What state is water at -12 C?
|
Solid
|
|
|
Mercury's melting point is -39 C and its boiling point is 357 C. What state is mercury at -10 C?
|
liquid
|
|
|
Ethanol's melting point is -117 C and its boiling point is 78 C. What state is ethanol when at 85 C?
|
Gas
|
|
|
Neon melts at -249 C and boils at -246. What's its state when at -247?
|
Liquid
|
|
|
What type of change is a state change -- physical or chemical?
|
physical
|
|
|
(T/F) The freezing and melting temperatures of a substance are the same temperature.
|
True
|
|
|
(T/F) The boiling & condensation temperatures of a substance are the same temperature.
|
True
|
|
|
A ______ is like a gas but refers to the gaseous stae of a substance that is usually a solid or liquid at room temperature.
|
Vapor
|
|
|
Similar to boiling, _______ occurs at ordinary or low temperature and has the same result -- liquid to gas.
|
evaporation
|
|
|
Ethyl alcohol boils at 78 C and water boils at 100 C. How could you separate them if they were combined together in a solution?
|
Keep the temperature between 78 and 100 C so that mostly gaseous alcohol would come out of the solution.
|
|
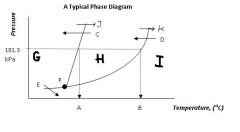
Regions G, H, & I are the ___, ___, ___ states of matter.
|
solid, liquid, gas
|
|
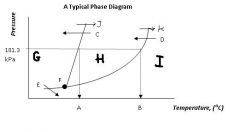
Point F, where all three states can exist in equilibrium, is called ....
|
the triple point
|
|
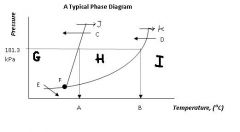
Arrow J and arrow K represent ____ and ____, respectively
|
melting
boiling |
|
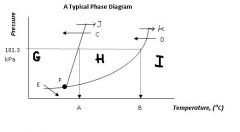
Arrow C and arrow D represent ____ and _____ respectively.
|
freezing
condensation |
|
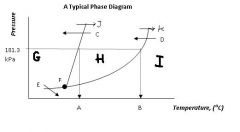
Arrow E represents....
|
sublimation (state change from solid to gas)
|
|
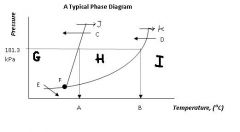
What's the siginificance of 101.3 kPa?
|
That's normal or standard atmospheric pressure.
|
|
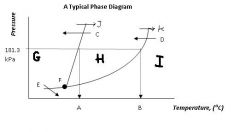
Locations A & B are the ____ ____ temperature and the ____ _____ temperature, respectively.
|
normal melting
normal boiling |

