![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
51 Cards in this Set
- Front
- Back
|
What is a cataract?
|
A disease characterized by the clouding of the ocular lens, and is a result of inadequate water and nutrient supply to fibre cells that make up the lens .
|
|
|
What is AQP0?
|
An aquaporin channel that makes up 60% of the fibre cell membranes in the ocular lens.
|
|
|
What does AQP0 do?
|
Active AQP0 regulates influx of water and nutrients into the lens.
Mutations of this activation pathway lead to the development of cataract. |
|
|
How does activation of AQP0 work?
|
AKAP2 binds to the C-terminal domain of AQP0 and
recruits PKA to phosphorylate its Ser235 residue, relieving inhibition of the channel by calmodulin (CaM) and allowing nutrient delivery to fibre cells. |
|
|
How is AKAP2 significant?
|
AKAP2 is the principal AKAP in the lens which anchors PKA at the fibre cell membranes in the lens outer cortex.
|
|
|
Clarifying, where does AKAP2 bind to on AQP0?
|
It binds at the C-terminal region.
|
|
|
What does phosphorylation of Ser235 on AQP0 do?
|
PKA phosphorylates AQP0 at Ser234, preventing CaM binding and opens the water pore.
- repulsion between negative charges of the CaM cleft and phosphorylated Ser235 results in displacement of CaM and opening of the pore. |
|
|
Does phosphorylation of AQP0 require AKAP2-mediated anchoring of PKA?
|
Why yes. Yes, it does.
|
|
|
What does fluid microcirculation help accomplish?
|
Lens transparency.
|
|
|
What do clathrin-coated pits do?
|
They control cell signalling by endocytosis of receptors in all eukaryotic cells.
|
|
|
What is the basic mechanism of CCP activity?
|
clathrin (polymerises in lattice), adaptor protein-2 (gathers receptors and clathrin),
GTPase dynamin (cleaves vesicle), lattice sheds, vesicle ready to travel |
|
|
What does Epsin1 do?
|
It is an adaptor protein that binds clathrin and AP-2 and is essential for clathrin-mediated endocytosis.
It helps in detection of the signal at the CCPs and is required for the ubiquitin-promoted pinching out of the pits. When Epsin1 was knocked out in the MOROck mutant, no effect was observed because there was no ubiquitin to detect. |
|
|
What is Smurf2? What is its main function?
|
It is a key E3 ubiquitin ligase in the endocytosis of the Mu opiod receptor (MOR).
Ubiquitination prolongs the surface lifetime of the clathrin coated pits. Smurf 2 ubiquitin ligase adds Ub to MOR after recruitment via beta-arrestin. |
|
|
Does ubiquitination prolong life of CCPs? How can lifetime be increased further?
|
Mutation of lysine residues can inhibit binding of ubiquitin to slow the endocytotic process even further.
In agonist-exposed cells, MOR and clathrin were shown to co-localize to form CCP. |
|
|
Are the CCPs ubiquitinated prior to the pinching off process?
|
Yes. Mutations to the dynamin molecule showed that CCPs are ubiquitinated prior to the pinching off process.
|
|
|
What does the ubiquitination of lysine residues in the first cytoplasmic loop of 7TMP result in?
|
It acts as an endocytic break release, controlling the amount of MOR in each CCP that is pinched off from the membrane.
|
|
|
What is coat-to-protein communication?
|
It is thought to control the endocytic pathways of CCPs. The idea of ubiquitin being a cargo specific regulator of all coat protein dynamics.
|
|
|
What is Akt?
|
It is a Ser/Thr protein kinase. It plays a role in glucose uptake (GLUT4), glycogen synthesis (GSK3-beta), and protein translation (mTOR).
|
|
|
How is AKT recruited to the membrane?
|
By PIP3 and it is activated by phosphorylation by PDK1 and mTORC2.
|
|
|
What is IP7?
|
It is an inositol phosphate synthesized from IP6 by IP6K1.
It inhibits protein localization by interactions with their PH domains - prevents recruitment by PIP3. Growth factor induced IP7 formation inhibits Akt signalling (no glycogen synthesis, no adipogenesis, and insulin resistance) |
|
|
In IP6K1 KO mice, what is expected?
|
They will have low insulin secretion from pancreatic beta-cells, but they still maintain normal glucose levels.
They display sustained insulin sensitivity. In insulin tolerance tests WT mice have prolonged blood glucose levels while KO show greater insulin-induced reductions of glucose. They show reduced fat accumulation when on a high fat diet and that they have improved glucose homeostasis. |
|
|
Where does IP7 exert its effects?
|
It inhibits Akt Thr308 phosphorylation and membrane translocation.
It competitively inhibits the PH domain (no inhibition when Akt has no PH domain). |
|
|
What decreases adipogenesis and increases fat oxidation?
|
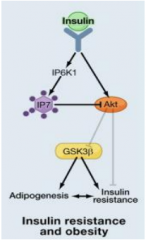
Absence of IP6K.
|
|
|
Is it true that IP6K1 KO mice have a hypersensitivity to insulin?
|
Yes. It protects against impaired glucose tolerance and hyperinsulinemia.
|
|
|
Respectively, what happens in Akt over-expression and Akt deletion?
|
Over-expression - leads to increased fatty acid oxidation in the liver.
Deletion - reduced fat accumulation through insulin resistance. |
|
|
Are there any issues with having an IP6K1 knockout?
|
Potential of tumors to form with the IP6K1 KO and decreased sperm formation as a side effect.
However, IP6K1 may be a good drug target for type 2 diabetes. |
|
|
What is TRAF6?
|
An E3 ligase responsible for IGF-1 mediated Akt ubiquitination.
However, TRAF6 is dispensable in EGF-mediated Akt ubiquitination. |
|
|
What is Skp2?
|
F-box protein (containing 10 Leu-rich repeats) that forms an SCF E3 ligase complex with Skp1, Cul1, and Rbx1, which is required for EGF-mediated Akt ubiquitination.
|
|
|
What type of ubiquitination does Akt undergo?
|
K63-linked, indicating Akt is a non-proteolytic substrate of Skp2-SCF.
|
|
|
What is the association of Skp2 and TRAF6 with their respective E3 ligase complexes dependent on?
|
The phosphorylation by various receptor tyrosine kinases. Treatment with phosphatase reduces TRAF6-Akt interaction and complete dissociation of Skp2-Akt.
|
|
|
Where are activated Akt's two phosphorylated sites?
|
In the A-loop and the hydrophobic motif (HM) of the C-terminal tail. Phosphorylation seems to be critical in its activation.
Skp2 silencing reduces EGF induced phosphorylation suggests Skp2 mediated ubiquitination is required for phosphorylation (and activation) of Akt by ErbB signalling. Ubiquitination is necessary for recruitment of Akt to the plasma membrane, but not for binding to PIP3. |
|
|
Is Skp2 involved in Akt activation? What are the effects if Skp2 is silenced?
|
Akt activation increases glucose uptake and lactate production in tumour cells (Warburg effect).
When Skp2 is silenced, cells exhibited reduced glycolysis and glucose uptake, indicating Skp2 is involved in Akt activation. Skp2 silencing slowed tumour growth. |
|
|
Herceptin in conjunction with Skp2 KO was able to do what to HER2-positive tumour cells?
|
Herceptin treatment alone impeded tumour growth, the combination of Skp2 silencing and Herceptin treatment resulted in tumour regression.
Skp2 overexpression is found in certain breast cancers, associated with poor prognosis and aggressive tumour growth (may be due to Akt activation without growth factor induction). |
|
|
What happens to PI3-Kinase signalling in tumors?
|
Signalling becomes enhanced by gene amplification, activating mutations, or inactivation of PTEN.
PI3Ks produce PIP3 in cells and stimulate proliferation, survival, and motility. |
|
|
Why is catalytic subunit p110-beta unique?
|
It can be activated by receptor tyrosine kinases and downstream GPCRs through direct binding of G-beta-gamma.
|
|
|
What is the development of PTEN-deficient prostate cancer dependent on?
|
On the activity of the p110beta-p85 dimer (referred to as PI3K-beta).
|
|
|
Why is it difficult to define the role of G-beta-gamma in activating p110-beta?
|
Transient nature of interactions between the two and the lack of a distinct G-beta-gamma binding motif that could be used to identify its target binding sites.
|
|
|
What site of p110-beta binds G-beta-gamma?
|
It is the non-conserved region in the linker between C2-domain and helical domains at residues 514-533.
Specifically, it is around Lys532/533. When mutated to Asp, neither mutant could be activated by G-beta-gamma. |
|
|
The PI3K signaling pathway strongly promotes cell survival, proliferation, and growth through the Ser/Thr kinase Akt (often altered in tumours). Does Akt activation require G-beta-gamma?
|
Yes. Mutants of Lys532/533 (bind G-beta-gamma) were unable to bind G-beta-gamma and resultantly, there was a complete loss of Akt phosphorylation.
GPCR inputs to PI3Kβ transmitted by Gβɣ are critical for PI3Kβ-mediated cellular transformation and enhancement of motility |
|
|
Where does p110-beta bind to on G-beta-gamma?
|
Residues 85-99 in the second blade of the beta-propeller binds several other different effectors.
Residues 31-45 in linker between N-terminal alpha helix and first blade of beta-propeller do not bind other G-beta-gamma effectors. (potential therapeutic target for specific G-beta-gamma activation of p110beta). |
|
|
Peptide inhibition of the p110beta-G-beta/gamma interaction is important for?
|
Blocking the proliferation and invasion of PTEN-null tumor cells. Growth of PTEN-null tumors depends on p110beta for growth and G-beta/gamma interactions with PI3Kbeta are critical for the growth and invasion of the PTEN-null tumors.
|
|
|
In presentation 5, how was proliferation of PC-3 cells prevented?
|
By the myr-p110beta peptide and pertussis toxin. Proliferation was unaffected if TGXX221 (a p110beta inhibitor) was present.
When mimicking interactions between macrophages and tumor cells during invasion, myr-p110β peptide blocked macrophage-dependent PC-3 cell invasion (fig. D) |
|
|
For translocation of GLUT4 to the plasma membrane, what is required?
|
The PIP3-Akt signaling pathway. Also, GLUT4 is insulin dependent (for reference).
|
|
|
What is PI3K's main role?
|
It phosphorylates PIP2 into PIP3 and creates DAG. PIP3 phosphatases hydrolyze PIP3 and negatively regulate PI3K-Akt signaling pathway.
|
|
|
What are the main PIP3 phosphatases?
|
SKIP, PTEN, and SHIP2 (not involved in regulation of insulin signaling in C2C12 cells). They are simultaneously expressed in C2C12 skeletal myocytes.
|
|
|
What does the suppression of SKIP do?
|
IT results in the specific regulation of insulin signaling by the suppression of SKIP. SKIP regulates PIP3 levels in an insulin-stimulation dependent manner whereas PTEN mainly controls PIP3 under basal conditions.
Suppression of SKIP increases Akt phosphorylation at Ser-473 and Thr-308 with insulin. |
|
|
What does suppression of PTEN result in?
|
Suppression of PTEN increases phosphorylation of Ser-473 of Akt under basal conditions, and increases phosphorylation of Ser-473 and Thr-308 with insulin stimulation.
SKIP and PTEN silencing increases GSK-3β and p70 S6 kinase phosphorylation. |
|
|
Do SKIP and PTEN influence PI3K independent signalling?
|
No, they do not influence PI3K independent signaling . SKIP and PTEN exert effects downstream of PI3K in the PI3K-Akt signaling pathway.
Suppression of SKIP did not affect insulin induced insulin receptor and ERK1/2 activation. |
|
|
What is AS160 and what effects does SKIP have on it.
|
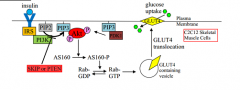
AS160 is a Rab-GTPase required for insulin-induced GLUT4 translocation to the plasma membrane and
glucose incorporation. AS160 phosphorylation by Akt is important in trafficking cells, including docking and fusion of GLUT4-containing vesicles. Attenuation of SKIP, not PTEN, resulted increased phosphorylation of AS160 (meaning, normal SKIP function leads to reduced AS160 phosphorylation) |
|
|
What are SKIP's main functions?
|
Regulate insulin-induced Akt phosphorylation, GLUT4 translocation, and glucose uptake in C2C12 cells. Potential pharmaceutical target aimed at diabetes treatment.
|
|
|
What are PTEN's main functions?
|
It mainly regulates GLUT4 localization at plasma membrane under basal conditions.
|

