![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
265 Cards in this Set
- Front
- Back
|
Where do primary messages go to when they first get a signal?
|
receptor integrated in plasma membrane. Primary messengers don't go into a cell.
|
|
|
What are the major secondary messengers?
|
cAMP, cGMP, Ca2+, IP3, DAG
|
|
|
What receptor does epi go to and what is the response?
Insulin? Epidermal growth factor? |
Epi
B-adrenergic (B-AR) receptor illicits energy store mobilization Insulin + Insulin receptor = increased glucose uptake EGF + EGF receptor= expression of growth-promoting genes |
|
|
Why are B-AdR's called serpentine receptors?
What happens when epi binds to it? |
this 7TM receptor protein snakes through the cell surface membrane 7 times
once the receptor is bound, cytoplasmic loops and carboxy terminus changes the conformation which activate G proteins |
|
|
Describe the broad pathway of activity once epi is bound to B-AdR.
|
G protein activated---->adenylate cyclase makes cAMP----->cAMP + ATP activates things like Protein Kinase A
|
|
|
What is the structure of G protein?
where is GDP bound? |
inactivated state
GDP bound to G protein aBy heterotrimer GDP is bound to P-loop of Ga |
|
|
What is the role of the H-R complex?
How is the GBy that stays once this is accomplished anchored to plasma membrane? |
H-R complex's job is to make GTP from GDP.
fatty acids bind GBy to plasma membrane |
|
|
Describe how a single bound H-R complex can cause amplification.
|
once the ligand is bound and one Ga dissociates from the GBy subunits it signals hundreds of G proteins to do the same and go from GDP to
GTP. |
|
|
What is a GPCR?
|
G-protein coupled receptors
another name for 7TM receptors |
|
|
Describe the enzyme adenylate cyclase
|
membrane bound
12 spanning domains, 2 intracellular catalytic domains Ga's surface that used to interact with the GBy dimer now interacts with catalytic site on AC and activates the enzyme |
|
|
What does amplified production of cAMP lead to?
What does that enzyme phosphorylate? |
cAMP activates PKA.
PKA phosphorylates Ser and Thr resides on MANY targets |
|
|
Name two examples of processes that PKA phosphorylation accomplishes?
|
PKA phosphorylates:
cAMP-response element binding protein which is a transcriptional activator of specific genes enzymes involved in glycogen metabolism (breakdown of glucose and regulation of glycogen production) |
|
|
How is Ga like a timer?
|
Once bound to AC, GTP has a period of time before it becomes hydrolyzed back into GDP and halting the transduction effects.
|
|
|
What type of G protein does angiotensin II utilize and what does it bind to in its GTP form?
|
Gaq
binds to enzyme phospholipase C |
|
|
What does the binding of Gaq to phospholipase C do?
|
Phospholipase C catalyzes PIP2 to IP3 and
also DAG |
|
|
How does the B-AdR get reset?
|
hormone dissociates
B-AdR kinase (also known as GRK2) phosphorylates C terminal tail of occupied B-AdR protein B-arrestin binds to phosphorylated receptor for further inactivation |
|
|
What does IP3 do in cells?
|
binds to ER membrane and causes rapid release of Ca2+ into cytoplasm
Ca2+, itself a messager, can bind proteins (calmodulin) or enzymes (PKC) and trigger smooth muscle contractions, glycogen breakdown and vesicle release. |
|
|
What causes Ca2+ to build up in ER of cells?
|
Ca2+-ATPase
|
|
|
What does DAG do in cells?
|
remains in plasma membrane
activates PKC (Ser/Thr protein kinase) works in tandem with IP3 because it needs Ca2+ |
|
|
What are Ca2+ levels inside and outside of cells?
|
low intracellular [Ca2+] to prevent phosphorylatd and carboxylated compounds from precipitating because these will form salts.
so only 100 nM of intracellular Ca2+ which is considerably lower than outside the cell This also makes small changes in Ca2+ readily detected |
|
|
Describe why Ca2+ is a good intracellular messenger given how it binds to proteins.
|
Ca2+ binds tightly to proteins
bind well to negative oxygen atoms on Glu and Asp and also to uncharged on Gln and Asn therefore Ca2+ can be coordinated to multiple ligands (6-8 oxygens) which enables it to cross-link different segments of a protein and induce significant conformational changes |
|
|
How do we detect Ca2+ flux?
|
Fura-2 binds to Ca2+ via oxygen atoms and changes its fluorescent properties which can be monitored in real time
|
|
|
Describe CaM (calmodulin).
|
17 kd protein
4 binding sites for Ca2+ CaM binds Ca2+ is concentration is >500nM member of EF hand protein family |
|
|
Describe the conformational changes that occur when CaM binds to Ca2+.
|
clamping down on Ca2+ causes hydrophobic surfaces to be exposed which will now bind with other proteins.
CaM clamps its hand down on a-helical regions through hydrophobic and ionic interactions |
|
|
Describe the insulin receptor.
|
dimer of 2 identical units
each one has an a and a B subunit. a's on outside of cell B's on inside Each B subunit is a tyrosine kinase |
|
|
what is a receptor tyrosine kinase?
|
the receptor for insulin. Each B subunit of the receptor is homologous to PKA, only instead of phosphorylating Ser/Thr, it phosphorylates Tyrosine via ATP and tyrosine kinase
|
|
|
what does the B subunit kinase of insulin have that makes it differ from PKA?
|
phosphorylates Tyr instead of Ser/Thr
has an activation loop in the center of structure that must be covalently modified to result in action. |
|
|
What happens when insulin binds to the a subunits outside of the cell?
|
forces the B subunit kinases inside the cell together, where the activation loop gets activated. The phosphorylation of Tyr causes a dramatic conformational change activating the Kinase Cascade
|
|
|
What are IRS's and where do they bind.
|
class of molecules called insulin-receptor substrates (IRS)
they bind to additionally phosphorylated sites from the action of tyrosine kinase on B subunit of insulin receptor |
|
|
What is a pleckstrin homology domain and where is it located?
|
Located on the amino end of IRS-1 and IRS-2, the pleckstrin homology domain binds phosphoinositide
|
|
|
What features exist in IRS-1 and IRS-2?
How do they work together? |
pleckstrin homology domain (binds phosphoinositide)
phosphotyrosine-binding domain (recognized by other proteins (SH2)) both domains act together to anchor IRS to phosphorylated receptor and plasma membrane. IRS also has 4 Tyr-X-X-Met sequences at C terminal (targes of insulin receptor tyrosine kinase) |
|
|
When tyrosine kinase phosphorylates the Tyr-X-X-Met 's on IRS's what does the IRS then become?
|
adaptor proteins
bind to lipid kinase and bring it to the membrane where it binds to its substrate, a membrane lipid. |
|
|
What is the most important class of kinases that are SH2 and bind to phosphotyrosine domain on IRS?
|
phosphoinositide 3-kinase
negative charges on phos-tyr interact with 2 Arg on SH2 protein this is a lipid kinase which phosphorylate PIP2 to PIP3 |
|
|
Describe lipid kinases.
|
heterooligomers made of 110 kd catalytic subunits and 85 kd regulatory subunits
Have SH2 domain on regulatory subunit which bind to IRS (recognizes the phospho tyr domain on IRS) and do the PIP2 to PIP3 thing. |
|
|
what is the name of the SH2 lipid kinase that does the PIP2 to PIP3 phosphorylation?
|
phosphatidylinositide -3 kinase
aka PI-3kinase |
|
|
Once you have PIP3 what happens?
|
PIP3 activates PDK-1
PDK-1 is a Ser/ Thr protein kinase with a PIP3 pleckstrin homology domain. Activated PDK-1 phosphorylates Akt-1 |
|
|
Why is Akt-1 different?
|
Not bound to plasma membrane
It migrates to intracellular targets and makes GLUT4 move to cell surface |
|
|
What happens when GLUT4 finally makes it to the cell surface?
|
glucose transported into cell
activation of glycogen synthesis and glycolysis |
|
|
Go through the entire insulin signaling pathway
|

|
|
|
Go through the epi signaling pathway.
|
Epi + B-AdR (binds to it)
Activated receptor leads to GaBy protein exchanging GTP for GDP Activated Ga dissociated and binds to AC AC increases cAMP cAMP activates PKA and other effectors |
|
|
What 3 classes of enzyme are important in terminating the insulin signaling?
|
--protein Tyr phosphatases
--lipid phosphatases --Protein Ser phosphatases |
|
|
what does protein Tyr phosphatase do?
lipid phosphatase? Protein Ser phosphatase? |
removes phosphates from insulin receptor
hydrolysis of PIP3 to PIP2 removes phosphate from kinases, Akt |
|
|
Describe the extracellular monomers of EGF receptor.
How are the extracellular monomers connect to intracellular tyrosine-kinase untis? |
Two extracellular monomers that bind TWO EGF ligands. The binding sites are far from eachother and the monomers only interact through dimerization arm.
single transmembrane helix for each monomer connects it to its intracellular B unit. |
|
|
Why doesn't EGF receptor dimerize and activate in the absence of EGF?
|
each receptor exists as a cyclic monomer and conformational changes (release of the dimerization arm) that bring the monomers together only occur after the ligand has bound to each monomer
|
|
|
What is Her2 receptor?
|
50% same as EGF receptor
no known matching ligand yet exists in extended conformation like bound EGF receptor Makes heterodimers with EGF receptors and creates cross phosphorylation Bad with cancer stuff |
|
|
Where does the cross-phosphorylation of the kinase occur once EGF binds to its receptor?
|
as opposed to insulin, where it occurs in activation loop, EGF cross phosphorylation occurs on C-terminal of kinase domain (this area is Tyr rich--up to 5 get phosphorylated)
|
|
|
What binds to phosphorylated Tyr's on C-terminal tails of kinase domains in EGF?
|
SH2 domain of Grb-2
Grb-2 has 3 domains, so the other 2 remaining SH3 domains bind Proline-rich regions of other proteins like Sos |
|
|
What binds to the 2 SH3 domains of Grb-2?
|
Proline rich regions of other proteins like Sos
|
|
|
What does Sos bind to?
How does Sos activate this protein? |
Ras-- a small G protein
Sos activates Ras by allowing the GDP to GTP switch in Ras |
|
|
What does Ras in its GTP form do?
What does that protein kinase then do? |
conformationally changes and binds to Raf
Raf conformationally changes and phosphorylates other proteins like MEK's |
|
|
What do MEK's do?
|
activate kinases called extracellular signal-regulated kinases (ERK's)
|
|
|
What do ERK's do?
|
phosphorylate substrates like transcription factors in nucleus and other protein kinases ultimately leading to changes in gene expression.
|
|
|
What are small G proteins also known as and describe them.
|
aka as small GTPases
monomeric and smaller that heterotrimic G proteins have a similar structure to Ga subunit Ras, Rho, Arf, Rab, Ran 20-25 kd |
|
|
Describe the full EGF signaling pathway
|

|
|
|
How are EGF signals terminated?
|
phosphatases get signaled by same signal that goes down stream to kinases that got phosphorylated (Ser, Thr, Tyr)
Ras has intrinsic GTPase which hydrolyzes GTP to GDP via GTPase Activating Proteins (GAPs) |
|
|
What are GAPs?
|
GTPase Activating Proteins
increase GTP hydrolysis in Ras as a signal terminating function |
|
|
Describe how viral oncogenes can lead to cancer.
|
viral proteins that resemble cellular proteins are missing the crucial Tyr (or other residue) that are part of the off switch in signal transduction pathways.
|
|
|
What are the 3 Ras genes found in mammals?
|
H-Ras
K-Ras N-Ras |
|
|
Explain tumor mutations in mammalian Ras genes.
|
GTPase activity is lost so Ras is trapped in the "on" setting and continual stimulation for cellular growth occurs.
|
|
|
Explain how glitches in tumor suppressor genes leads to cancer.
|
tumor suppressor genes (usually phosphotases) are normally present in a cell but can get deleted or damaged
|
|
|
What is EGFR and which cancers is it overexpressed in?
|
Epidermal Growth Factor Receptors
Breast Ovarian Colorectal |
|
|
How do monoclonal antibodies treat cancer?
|
Monoclonal AB's target EGFRs by competing with EGF
Keeps the grow and divide signal limited. |
|
|
What is cetuximab (Erbitux)?
|
Monoclonal AB for colorectal cancer. Competes with EGF for spots on the receptor and also inhibits dimerization and initiation of grow and divide signal through steric hindrance.
|
|
|
How does trastuzumab (Herceptin) work to treat breast cancer?
|
Trastuzumab (Herceptin) blocks Her2 receptors which can dimerize even without a ligand.
Only works with Her2 breast cancers acquired resistance is a problem though |
|
|
Describe the signaling pathway for Angiotensin II.
|
Angiotensin II binds to its 7TM receptor
Activates Gaq to it's GTP form Gaq (GTP) activates phospholipase C Phospholipase C cleaves PIP2 into IP3 and DAG IP3 binds to ER membrane and Ca2+ is released DAG binds to PKC (which is maximally activated by DAG and Ca2+) |
|
|
What is glycolysis?
|
sequence of reactions that metabolizes one molecule of glucose to two molecules of pyruvate with the concomitant net production of 2 molecules of ATP.
|
|
|
what happens to glucose in aerobic conditions?
limiting O2 conditions? anaerobic conditions? |
aerobic--pyruvate enters mitochondria, oxidation to CO2 and H20
limiting O2--pyruvate becomes lactate anaerobic--pyruvate become fermented to ethanol |
|
|
What are the first 3 steps in stage 1 of glycolysis?
|
phosphorylization
(Hexokinase phospho's glucose 6) Isomerization (Phosphoglucose isomerase isomerizes glucose 6-phosphate to fructose 6-phosphate) 2nd Phosphorylization (Phosphoructokinase catalyzes ATP's phospho of Fructose 6-phosphate to turn it into 1,6 bisphosphate) |
|
|
What's the main goal of stage 1 of glycolysis?
|
trap the glucose in the cell and form a compound tha can be readliy cleaved into phophorylated three-carbon units
|
|
|
What is the main goal of stage 2 in glycolysis?
|
cleavage of the fructose 1,6-bisphosphate into 2 three carbon fragments
|
|
|
What is the goal of stage 3 of glycolysis?
|
ATP is harvested when the three-carbon fragments are oxidized to pyruvate
|
|
|
What does phosphorylating the glucose molecule as soon as it gets into the cell accomplish?
What enzyme catalyzes ATP to phosphorylate here and what does this enzyme require to do so? |
phosphorylating glucose destabilizes the glucose and traps it there.
hexokinase, Mg2+ |
|
|
Which carbon gets phosphorylated upon entering the cell?
|
C-6 OH
|
|
|
what happens conformationally when hexokinase binds to glucose?
|
conformational changes:
--2 lobes move together 8 A --12 degree rotation --cleft closes on glucose and only the 6 hydroxymethyl group is exposed Induced fit causes: --nonpolar environment excluding water around glucose Makes 6-OH non-polar and thus more phophorylatable and also excludes water from binding as a substrate |
|
|
What is a general feature of kinases?
Why is this significant? |
substrate induced cleft-closing
prevents these enzymes from becoming ATPases and making ADP + Pi |
|
|
What reaction does phosphoglucose isomerase catalyze?
|
glucose 6-phosphate ---> fructose 6-phosphate
a conversion from an aldose to a ketose |
|
|
What reaction is catalyzed by phophofructokinase?
|
catalyzes ATP to phosphorylate fructose 6-phosphate and turn it into fructose 1,6-bisphosphate
|
|
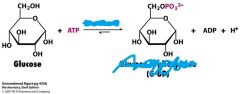
What is the enzyme and product for this reaction?
|
Hexokinase
Glucose 6-phosphate |
|
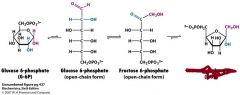
What is the enzyme for this reaction and product?
|
phosphoglucose isomerase
|
|

what is the enzyme and product of this reaction?
|
phosphofructokinase
Fructose 1,6-bisphosphate |
|
|
How does stage 2 start out in glycolysis?
What enzyme is involved here? |
splitting of fructose 1,6 bisphophate into glyceraldehyde 3-phosphate (GAP) and
dihydroxyacetone phosphate (DHAP) We are now dealing with 2 3-Carbon dudes. aldolase |
|
|
At the beginning of stage 2, which of the 3-Carbon products are on the direct pathway?
What has to happen to the other one in order to not lose ATP? |
GAP is on direct pathway
DHAP must be converted to GAP via triose phosphate isomerase (TPI or TIM) |
|
|
Why is the isomerization from glucose 6 to fructose 6 to 1,6 bisphosphate so important?
|
creates 2 3-carbon badboys, one which can easily be converted to the useful GAP and thus only requires 1 pathway.
|
|
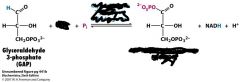
What is needed for this reaction, what is the enzyme and what is the product?
|
NAD+
Glyceraldehyde 3-phosphate dehydrogenase 1,3-Bisphosphoglycerate |
|
|
By the end of stage two, how much ATP has been invested and how much has been made?
|
2 invested
0 made |
|
|
what's the deal with 1,3-bisphosphoglycerate that is going to eventually lead to the production of ATP?
|
It is an acyl phosphate (mixed anhydride of phosphoric and carboxylic acid)
This makes it have high phosphoryl-transfer potential to ADP to make ATP |
|
|
Describe the two processes of the reaction catalyzed by 3-phosphate dehydrogenase.
What is the favorability of each and the standard free energy change? |
1). Oxidation of aldehyde to carboxylic acid by NAD+
2). Joining of carboxylic acid and Pi to form the acyl-phosphate group Step one is thermodynamically favored -12 kcal/mol step two is unfavored +12 kcal/mol |
|
|
What is the enzyme that catalyzes the transfer of Pi to ADP from 1,3-biphosphoglycerate?
|
Phosphoglycerate kinase
|
|

What is the enzyme and product from this reaction?
|
Phosphoglycerate kinase
3-phosphoglycerate |
|
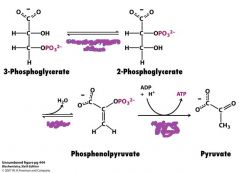
What are the enzymes involved in this reaction?
|
Phosphoglyerate mutase
Enolase Pyruvate kinase |
|
|
what does a mutase do and what does it do in glycolysis?
|
intramolecular shift of a chemical group, like say, a phosphoryl group.
in glycolysis phosphoglycerate mutase shifts a phosphoryl from 3 to 2 position |
|
|
why does phosphenolpyruvate (PEP) have such a high transfer potential?
|
the phosphoryl group traps this molecule in the enol (more unstable form) so it wants to give away the phosphate to become a happy little ketone (pyruvate).
|
|
|
What's the standard free energy change in hydrolysis of PEP
|
-15 kcal/mol
|
|
|
What is the net reaction of glucose into pyruvate?
|
glucose + 2 Pi + 2 ADP + 2 NAD+ -------->2 pyruvate + 2 ATP + 2 NADH + 2 H+ + 2 H2O
2 molecules of ATP overall energy released is -23 kcal/mol |
|
|
What are 3 potential fates of pyruvate?
|
conversion into
ethanol Lactate acetyl CoA |
|
|
Describe what happens when pyruvate is transformed to ethanol.
|
pyruvate decarboxylase (enzyme) kicks off CO2 from pyruvate
Now you have acetaldehyde Enzyme alcohol dehydrongenase comes in and positions the carbonyl C and polarizes it with it's zinc in order for NADH to transfer a H+ to it regenerating vital NAD+ and ethanol. |
|
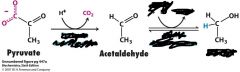
What are the enzymes and necessary things for this reaction?
what's the product? |
pyruvate decarboxylase
alcohol dehydrogenase (need NADH to give H+ to ethanol |
|
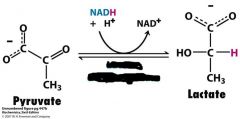
What's going on in this reaction and what is the enzyme needed?
|
lactate dehydrogenase
anaerobic conditions |
|
|
What is the fructose 1-phosphate pathway?
|
fructokinase phosphorylates fructose into fructose-1 phosphate.
Then, fructose 1-phosphate aldolase splits fruct1-Pi into Glyceraldehyde and DHAP Glyceraldehyde gets phosphorylated by triose kinase (and ATP) to GAP which is obviously a glycolysis intermediate |
|
|
What are the 4 steps of galactose to turn it into glucose 6-phosphate?
|
1). galactokinase uses ATP to phosphorylate galactose into galactose 1-phosphate
2). Galactose 1-phosphate gets UDP using galactose 1-phosphate uridyl transerase. Makes UDP galactose and Glucose 1-phosphate 3). UDP galactose-4-epimerase inverts a hydroxyl to make UDP-glucose 4). phosphoglucomutase shifts the phosphate to Glucose 6-phosphate |
|
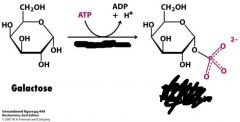
What is this the first step in?
What enzyme is needed? |
First step in making galactose into glucose 6-phosphate
needs galactokinase and ATP |
|
|
What are the two substances in muscles that tell the regulatory enzymes what to do?
|
ATP and AMP
|
|
|
What allosterically inhibits phosphofructokinase (PFK-1)?
|
ATP binds to regulatory sites on PFK-1 and lowers it affinity for fructose-6-Pi
(PFK-1 binds to fructose-6-Pi to allow ATP to phosphorylate it to give fructose-1,6-bisphosphate in stage 1) |
|
|
What does low levels of ATP do to PFK-1?
|
PFK-1 is uninhibited.
low levels of ATP want glycolysis to happen so there is no inhibition |
|
|
What do high levels of ATP do to PFK-1?
|
inhibit PFK-1.
high levels of ATP means no more glycolysis is needed so halts the pathway by inhibiting PFK-1 from phosphorylating fructose-6-Pi |
|
|
Where are the regulatory sites on PFK-1?
|
very distal from the catalytic sites.
this is definitely an allosteric regulation |
|
|
What does increasing AMP do to PFK-1 inhibition?
|
Increasing the AMP levels means it dilutes the mix of ATP / AMP ratio.
lowers inhibition (makes it look like we need more ATP) |
|
|
What does increased levels of citrate indicate to the regulation of PFK-1?
|
Citrate indicates that there is alot of energy around so it increases the inhibition of PFK-1
|
|
|
What does a lowered ATP: AMP ratio do?
|
stimulates PFK-1
stimulates glycolysis |
|
|
Why does decreasing pH augment the ATP inhibition of PFK-1?
|
If pH is decreasing it indicates muscle is functioning anaerobically and forming lactic acid. Stops glycolysis to protect it from too much of this
|
|
|
Why is AMP used instead of ADP as an allosteric measurer?
|
when ATP is getting used up FAST, the body will make it by combining 2 ADP and AMP is a byproduct
(2 ADP---> ATP + AMP) This shows the cell is in a low energy state small changes in ATP lead to BIG changes in AMP so it is a sensitive controller |
|
|
Why is hexokinase an example of an enzyme with immediate negative feedback inhibition?
|
It's product, Glucose-6-Pi, is its inhibitor.
|
|
|
How do high levels of fructose6-Pi inhibit hexokinase even though product is down the pathway?
|
fructose-6-Pi is in equilibrium with glucose-6-Pi which is an inhibitor to hexokinase.
When PFK-1 is inhibited and not phosphorylating Fructose-6-Pi it is at high levels and thus so will glucose-6-Pi and be inhibiting hexokinase. |
|
|
how is pyruvate kinase allosterically inhibited in muscle cells?
|
ATP inhibits
transanimation of alanine signals that building blocks are abundant |
|
|
What is an activator for pyruvate kinase in muscle cells?
|
Fructose 1,6-bisphosphate
|
|
|
how do these regulation effectors differ from muscle to liver cells?
ATP pH citrate |
ATP is same for PFK for muscle and liver
pH--no signaling effects in liver because there is no lactate produced there citrate--inhibits PFK and enhances ATP effect |
|
|
What is Fructose 2,6-bisphosphate and what does it do in liver?
|
outside allosteric regulator from PFK-2
when concentrations of Fructose 6-phosphate rise (abundantly, like after a big ice cream cone), this signaler stimulates PFK (and reduces the inhibitory effects of ATP) |
|
|
What signal molecule does PFK-2 make?
|
Fructose 2,6-bisphosphate
|
|
|
what is glucokinase?
|
specialized isozome of hexokinase
50-fold lower affinity than hexokinase at work in abundant glucose moments to make glycogen and fatty acids because the liver is the blood sugar guru, it has this enzyme which is not inhibited by glucose 6-Pi assures brain and muscle get taken care of first and that glucose is not wasted when it is high |
|
|
How do the L and M isomeric forms differ of pyruvate kinase?
|
L form is controlled by reversible phosphorylation (M is not)
when blood glucose is low cAMP is increased and this results in phosphorylation of Lpyruvate kinase which inhibits it and thus saves glucose for brain and muscle |
|
|
What are the blood glucose transporters and where do they reside?
|
GLUT1 and 3
all cells GLUT2 liver and pancreatic B-cells GLUT 4 muscle and fat GLUT5 small intestine (fructose) |
|
|
Which type of GLUT has a high Km and what does that mean?
|
GLUT2, located in liver and pancreatic B-cells, has a high concentration gradient to overcome and thus only enters these cells when blood glucose levels are soaring
|
|
|
What does gluconeogenesis do?
|
converts pyruvate into glucose
|
|
|
what are the major non-carbohydrate precursors in gluconeogenesis and where do they enter?
|
lactate--pyruvate
amino acids--OAA glycerol--DHAP |
|
|
What are the releases of free energy at each of the 3 irreversible steps of glycolysis which show why glucose ---->pyruvate is highly favored?
|
hexokinase
^G= -8 kcal/mol PFK ^G= -5.3 pyruvate kinase ^G= -4 kcal/mol |
|
|
What are the equations for the 3 irreversible steps in glycolysis?
|
hexokinase
glucose + ATP ---> glucose 6-Pi + ADP PFK fructose 6-Pi +ATP--> fructose 1,6-bisphosphate + ADP pyruvate kinase PEP + ADP--->pyruvate + ATP |
|
|
What's the gluconeogenesis bypass reaction to form PEP?
what enzymes are utilized? |
pyruvate + CO2 + ATP + H2O--->OAA + ADP + Pi +2H
enzyme is pyruvate caroxylase occurs in mitochondria OAA + GTP--->PEP +GDP + CO2 enzyme is PEP carboxylkinase occurs in cytoplasm this is the carboxlation of pyruvate using ATP needs biotin enzyme is pyruvate caroxylase |
|
|
What's the gluconeogenesis bypass reaction to form fructose 6-Pi from fructose 1,6-bisphosphat?
What enzyme is utilized? |
fructose 1,6-bisphosphate + H2O -----> fructose 6-Pi + Pi
frustose 1,6-bisphosphatase |
|
|
What is the gluconeogenesis bypass reaction for glucose 6-Pi to glucose?
What enzyme is utilized? |
glucose 6-Pi + H20 ---> glucose + Pi
Need T1, T2, T3, SP (Ca2+), G6Pase proteins |
|
|
Why are carboxylations and decarboxylations necessary to make PEP from pyruvate?
Describe them in this rxn. |
decarboxylation and carboxylation is required in this step because it's really endergonic to phosphorylate pyruvate to get it to stick in the unstable enol form of PEP
ATP provides energy to put CO2 on pyruvate and then the CO2 is removed to form PEP |
|
|
Why must OAA be transported out of mitochondrial matrix as malate?
What's the rxn that is taking place inside the mitochondria? |
pyruvate carboxylase is a mitochondrial enzyme.
reduction of OAA to make it malate OAA + NADH + H--->malate NAD+ |
|
|
Where does the oxidation of malate take place and what does it generate?
|
cytoplasm
malate + NAD+---->OAA + NADH + H NADH used in other glycolosis steps |
|
|
How is fructose-1,6-bisphospatase inhibited?
How is it activated? |
AMP and fructose 2,6-bisPi
citrate |
|
|
What inhibits pyruvate kinase?
What activates it? |
ATP, Ala and phosphorylation inhibits
F-1,6-bisPi activates it |
|
|
What inhibits pyruvate carboxylase?
What activates it? |
ADP inhibits
Acetyl CoA stimulates (needs OAA) |
|
|
What inhibits PEP carboxylase?
|
ADP
|
|
|
When is gluconeogenesis favored?
|
When the cell is rich in biosynthetic precursors and ATP. (No immediate energy needs)
|
|
|
What is the enzyme that phosphorylates fructose 6-Pi to make fructose 2,6 bisphosphate when blood sugar is high?
|
PFK-2
|
|
|
What enzyme catalyzes the formation of Fructose 6-Pi from fructose 2,6-Pi when blood sugar levels are low?
What does fructose 2,6-Pi do again? |
fructose bisphosphatase 2
Fructose 2,6-bisPi stimulates PFK to increase glycolosis |
|
|
What's interesting about PFK-2 and fructose biphosphotase 2?
|
They are in the same enzyme.
This bifunctional enzyme has regulatory domain, kinase domain and phosphotase domain PFK-2 domain looks like Adenylate kinase with P loop NTPase domain FBPase2 looks like phosphoglycerate mutase |
|
|
What signals phosphorylation of PFK-2?
|
low glucose and increased levels of glucagon (fasting state)
|
|
|
what signals the dephosphorylation of PFK-2?
|
increased glucose levels in the blood.
PFK-2 is activated (FBPase2 inhibited) so it phosphorylates fructose 6-Pi into fructose 2,6 bisPi which stimulates PFK which ramps up glycolysis |
|
|
What does insulin stimulate?
|
PFK
pyruvate kinase F2,6-BP |
|
|
What does glucagon control?
|
inhibits PFK, pyruvate kinase and F-2,6 BP
increases expression of PEP carboxykinase and F1,6 biphosphatase (two key gluconeogenesis enzymes) |
|
|
How does Liver hexokinase differ from muscle hexokinase?
|
Liver hexokinase operates essentially the same as it does in muscle, however, it has a special isozyme called glucokinase which is not inhibited by high levels of glucose 6Pi.
with its lower binding affinity it gives brain and muscle priority and also conserves waste |
|
|
Is liver pyruvate kinase more or less active when it is phosphorylated?
Does M pyruvate kinase utilize reversible phosphorylation? |
Less active
No, M form does not utilize reversible phosphorylation |
|
|
What is the Km for liver and pancreatic B-cells for GLUT 2?
What is the Km for muscle and fat cells for GLUT4? |
15-20 mM (liver and pan)
5 mM (muscle and fat) |
|
|
where does gluconeogenesis generally occur?
What are the non-carb precursors? |
liver and kidney cortex
lactate enter at pyruvate amino acids enter at OAA glycerol enters at DHAP |
|
|
What are the 4 new enzymes of gluconeogenesis?
And what are the 2 enzymes in the mitochondria that convert OAA to and from malate? |
pyruvate carboxylase
PEP carboxykinase Fructose 1,6-bisphosphatase Glucose 6- phosphatase NADH-linked malate dehydrogenase NAD+-linked malate dehydrogenase |
|
|
What are the 5 proteins needed in the ER hydrolysis of Glucose-6Pi into Glucose?
|
T1
T2 T3 SP (Ca2+) G6Pase |
|
|
What is the funciton of the citric acid cycle?
|
To harvest high energy electrons through a series of oxidation-reduction reactions.
These high energy electrons fuel the synthesis of ATP. |
|
|
What is a hydride ion?
|
H:-
has 2 e- |
|
|
What is NAD+
|
major electron carrier
NAD+ is oxidized form and ring N carries + charge Accepts hydride ion and its 2 eletrons |
|
|
Where do the electrons captured in TCA cycle come from?
|
Acetyl CoA
|
|
|
What is oxidative decarboxylation of pyruvate?
|
links glycolysis to TCA cycle
pyruvate + CoA + NAD+ ---> acetyl CoA + CO2 + NADH |
|
|
What is the pyruvate dehydrogenase complex?
What are the 3 steps that it does? |
highly integrated complex of 3 enzymes
decarboxylation of pyruvate (kicking off CO2) oxidation of hydroxyethyl group transfer of acetyl group to CoA to form Acetyl CoA |
|
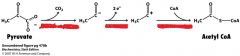
Describe what is happening here.
|
PDH complex activity
decarboxylation oxidation Transfer to CoA |
|
|
What are the 3 enzymes and their activities of PDH complex?
|
E1 pyruvate dehydrogenase
(oxidative decarboxylation) E2 dihydrolipoyl transacetylase (transfer of acetyl group E3 dihydrolipoyl dehydrongenase (regeneration of oxidized lipoamide) |
|
|
What does E1 do?
|
oxidative decarboxylation of pyruvate
|
|
|
What does E2 do?
|
transfers acetyl group from acetyllipoamide to CoA
|
|
|
What does E3 do?
|
oxidation of dihydrolipoamide to regenerate lipoamide
|
|
|
What are the 5 cofactors of PDH complex?
|
TPP
lipoic acid FAD CoA NAD+ |
|
|
What is the ultimate goal of all the E enzymes and PDC reactions?
|
Convert pyruvate into Acetyl CoA
|
|
|
What is the reaction that utilizes citrate synthase?
|
OAA+ acetyl CoA + h2o ---->citrate + HS-CoA (thioester high energy bond)
|
|
|
Describe how citrate synthase works.
|
OAA binds first followed by acetyl CoA
OAA binding creates binding site for acetyl CoA |
|
|
Describe the isomerization of citrate.
|
Need to reposition the hydroxyl group.
citrate---> cis-aconitate + h2o--->isocitrate dehydration and then hydration enzyme is aconitase |
|
|
Describe aconitase?
|
4 Fe and 4 sulfide (3 Cys S) non-heme enzyme
Fe binds to COO- and OH- parts of citrate for the dehydration and hydration rxns that take place |
|
|
What is the first oxidation-reduction reaction in TCA cycle and what is the enzyme utilized?
|
Isocitrate + NAD+-----> oxalosuccinate + NADH + H-->
a-ketoglutarate isocitrate dehydrogenase |
|
|
Describe oxasuccinate.
|
unstable B-ketoacid that while bound to isocitrate dehydrogenase loses a CO2 to form a-ketoglutarate
|
|
|
What is the 2nd ox-redux reaction of TCA cycle and what is the enzyme utilized?
|
a-ketoglutarate + NAD+ +CoA ---->succinyl CoA + CO2 + NADH
a-ketoglutarate dehydrogenase complex |
|
|
What is the a-ketoglutarate dehydrogenase complex?
|
3 distinct enzymes with similar mechanism to PDH complex
|
|
|
what does the cleavage of the thioester bond in the citrate synthase reaction give fuel for?
|
synthesis of 6-carbon citrate from 4-carbon OAA and 2- carbon fragment
|
|
|
what does the cleavage of the energy-rich thioester bond in succinyl CoA provide energy for?
|
powers the putting a Pi on GDP (or ADP)
Remember GTP is an important factor in signal transduction. |
|
|
What is the enzyme involved in breaking the thioester bond of Succinyl CoA and putting a Pi on GDP
|
succinyl CoA synthetase
|
|
|
What is the Rossman fold?
|
Amino -terminal end of a subunit of succinyl-CoA synthetase
binds ADP area of succinyl CoA |
|
|
What is the ATP grasp area of succinyl CoA synthetase?
|
area that binds and activates GDP to GTP
|
|
|
What are the 3 steps in how succinate (4-carbon guy) is transformed back into OAA?
|
oxidation
succinate + E-FAD--> fumerate + E-FADH2 succinate dehydrogenase hydration fumarate + H2O-->L-malate fumarase oxidation malate + NAD+ --> OAA+ NADH + H malate dehydrogenase |
|
|
which reaction is FAD the H acceptor in?
Why? |
Succinate to fumarate
(succinate dehydrogenase is enzyme) Free energy change is insufficient to reduce NAD+ |
|
|
What are the two Fe-S enzymes in TCA cycle?
|
aconitase--participates in dehydration / hyrdration to make isocitrate
succinate dehydrogenase--participates in donating electrons to electron transport chain via these Fe-S groups to CoQ |
|
|
Where is succinate dehydrogenase located?
Why is this significant? |
inner mitochondrial membrane
(this is unique to this enzyme) FADH2 produced from this enzyme doesn't dissociate from succinate dehydrogenase but rather transfers the electrons to CoQ which passes electrons to molecular Oxygen directly |
|
|
What is the reaction that malate dehydrogenase catalyzes?
|
malate + NAD+ ---> OAA + NADH + H
|
|
|
How many ATP are generated per oxidization of NADH?
How many ATP are generated from the oxidation of FADH2? |
~2.5
~1.5 |
|
|
How many high energy ~P are generated from the 3 NADH and 1 FADH made in the TCA cycle?
How many Pi are formed directely in the cycle? How many total ATP produced from one acetyl unit? |
9
1 total of 10 |
|
|
What is a metabolon?
|
a word to describe the physical connectivity of the enzymes of the citric acid cycle that allows for efficiency through substrate challenging
|
|
|
Why is citric acid cycle only an aerobic mode?
|
NAD+ and FAD are only regenerated once they dump their electrons on O2.
Hence you need O2. |
|
|
What is the key irreversible step in regulation of TCA cycle?
|
allosteric and reversible phosphorylation of PDH complex
|
|
|
Because the transformation from pyruvate to acetyl CoA is irreversible, what are the two choices for the carbon atoms once they've been committed?
|
TCA cycle or lipid biosynthesis
|
|
|
What things regulate the PDH complex?
|
High concentrations of reaction products
(acetyl CoA inhibits E2/transacetylase directly) (NADH inhibits E3 / dihydrolipoyl dehydrogenase) High energy charge tells enzymes no need to metabolize more glucose |
|
|
How is PDH complex regulated?
|
When high energy / reaction products are there phosphorylation of PDH (E1)occurs shutting it off.
So when it needs to be turned back on a specific phosphatase dephosphorylizes PDH |
|
|
Describe what happens when a muscle starts exercising to switch the PDH complex back on.
|
muscle starts working, using ATP so ADP and pyruvate levels start to rise as glycolysis goes.
The rising levels of ADP and pyruvate inhibit kinase. At same time Ca2+ (product of muscle contraction) tells phosphatase 'GO" |
|
|
How does epi in liver regulate the phosphatase that is in charge of PDH complex?
|
epi binds to the a-adrenergic receptor which activates phosphatidylinositol pathway (think angiotensin II pathway)
Remember how it opens Ca2+ channels on ER via IP3? And now that Ca2+ activates the phosphatase that dephosphorylates PDH complex allowing it to be active |
|
|
How does insulin regulate the phosphatase that is in charge of PDH complex?
|
insulin tells tissues capable of fatty acid synthesis (liver and adipose tissues) that we have a fed state and it stimulates the phosphatase to make acetyl CoA
|
|
|
What is the first primary control point of TCA cycle?
How is it allosterically controlled? |
isocitrate dehydrogenase
ADP stimulates affinity for substrate (NAD+, Mg2+, ADP mutually cooperative) ATP and NADH are inhibitory because they kick out the necessary NAD+ and FAD |
|
|
What is the second primary control point of TCA cycle?
How is it allosterically controlled? |
a-ketoglutarate deyhdrogenase complex
it's products succinyl CoA and NADH and high energy charge inhibit So high ATP decreases the rate of the cycle |
|
|
Connect the dots between how citrate made in TCA cycle correlates with regulating glycolysis.
|
inhibition of isocitrate dehydrogenase leads to a build up of citrate which spills into cytoplasm and signals PFK-1 to halt glycolysis
|
|
|
What does a build up citrate lead to?
a build up of a-ketoglutarate? |
citrate used as a source of acetyl CoA in fatty acid synthesis
a-ketoglutarate is used as amino acid precursor purine base precursor |
|
|
What is 3rd control point of TCA cycle?
How is it allosterically regulated? |
citrate synthesis in bacteria
ATP inhibits by increasing the Km for acetyl CoA |
|
|
In addition to making ATP, the TCA cycle also makes a lot of biosynthesis precursors.
What does succinyl CoA make? What does a-ketoglutarate and OAA make? |
Succinyl CoA is the porphyrin carbons (think heme)
OAA-->transamination --> Asp amino acids, purines, pyrimidines, glucose a-KG-->transamination--->Glu amino acids, purines |
|
|
How is OAA replenished for use in the TCA cycle?
|
Pyruvate uses pyruvate carboxylase (biotin dependent)
Energy high? gluconeogenesis Energy low? OAA replenishes TCA cycle metabolism is going to make ATP so it puts it in the TCA cycle |
|
|
What types of genes are fumarase and succinate dehydrogenase?
What type of cancers do mutations on these genes lead to? |
tumor suppressor genes
accumulation of fumarase aids HIF-a leiomas and kidney tumors succinate dehydrogenase lead to adrenal gland tumors (pheochromocytomas) and HIF-a induction |
|
|
What are the component names for E1, E2 and E3 in PDH complex and what does each one do?
|
E1--pyruvate dehydrogenase component
decarboxylates pyruvate by combining it with TPP to make hydroxyethyl TPP also oxidizes hydroxyethyl TPP by combining it with lipoamide to make acetyllipoamide E2-dihydrolipoyl transacetylase forms acetyl CoA by combining CoA with acetyllipoamide E3- dihydrolipoyl dehydrogenase reoxidizes lipoamide and in doing so transfers to electrons to FAD and eventually to NAD+ |
|
|
what is oxidative phosphorylation?
|
high transfer potential electrons from NADH and FADH2 are used to reduce molecular Oxygen to water and a large amount of free energy is released which can be used for ATP synthesis
|
|
|
What are the 3 electron driven proton pumps?
|
NADH-Q oxidoreductase--Complex I
Q-cytochrome c oxidoreductase--Complex III cytochrome c oxidase--Complex IV |
|
|
What is the electon motive force?
proton motive force? What do they create across the membrane? |
electron motive force is electrons made in TCA cycle
proton motive force is the flow of H back into cell a transmembrane electrical potential is generated |
|
|
What is ATP synthase?
|
ATP synthesizing assembly driven by flow of protons back into mitochondrial matrix
|
|
|
Where does the TCA cycle take place?
|
matrix of mitochondria
outer mem intermembrane space inner membrane matrix |
|
|
Where does oxidative phosphorylation take place?
|
inside the mitochondrial inner membrane
cristae provide more surface area for this to take place |
|
|
what is a mitochondrial porin?
|
pore forming protein
Voltage Dependent Anion channel permeable to metabolites |
|
|
What is N side vs P side?
|
N=negative matrix side where it becomes negative
P side is cytoplasm side of inner membrane (named cytoplasm because stuff from cytoplasm can come in due to permeability of outer mitochondrial membrane) P=postive where the protons are pumped out to make it positive. |
|
|
what do the large family of transporters have to shuttle across inner mitochondrial membrane?
|
ATP, citrate, pyruvate
The inner membrane does NOT have the pores like the outer membrane |
|
|
Does NADH have a higher or lower affinity for electrons than O2?
This makes it a strong____________ agent. |
lower affinity --means it's poised to donate electrons
strong reducing agent |
|
|
What are the delta E` (reduction potentials) of NAD+ and 1/2O2?
|
-0.32 (strong reducing agent, low affinity, wants to donate e's)
+0.82 (strong oxidizing agent, high affinity, wants to accept e's) |
|
|
What kind of reaction has a positive delta E` and a negative delta G`?
|
exergonic
|
|
|
What is the equation of oxidative phosphorylation and what are the Free Energies associated with it?
|
1/2O2 + NADH + H----> H2O + NAD+
Delta E` +1.14 V Delta G` -52.6 kcal/mol |
|
|
What is the name for Complex II?
How is it different? Where does it enter it's electrons in the chain? |
Succinate Q-reductase
Not a proton pump. Brings the electrons from FADH2 from TCA cycle in and inserts them at Q-cytochromoxireductase |
|
|
Describe NADH-Q oxidoreductase.
|
Complex I
oxidizes NADH, reduces FMN to FMNH2 and takes its electrons to Q via Fe-S clusters |
|
|
Describe Q cytochrom c oxidoreductase.
|
small protein
Complex III oxidizes Q and takes it electrons to Cyt c |
|
|
Describe cytochrome c oxidase.
|
Complex IV
oxidizes cyt c and takes its electrons to O2 |
|
|
What is a respirasome?
|
supramolecular complex which facilitate the rapid transfer of substrate and prevent the release of reaction intermediates.
|
|
|
How many oxidation states does Coenzyme Q have?
What does it have in fully oxidized state? semiquinone state? Fully reduced form? |
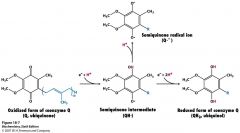
3 oxidation states
fully oxidized has 2 ketones semi state has one OH and one O. radical fully reduced state has 2 OH's |
|
|
What is flavin and who donates electrons to it?
|
FMN -- has same isoalloxazine ring as FAD
Occurs at beginning when NADH donates 2 electrons and forms FMNH2 (reduced) |
|
|
What is the equation for complex I?
Describe what happens here. |
NADH + Q +5Hmatrix---> NAD+ +Qh2 + 4Hcytoplasm
NADH donates 2 e's to FMN making FMNH2. FMNH2 gives the electrons to series of Fe-S clusters Fe-S gives electrons to CoQ |
|
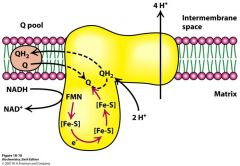
Describe what is happening in this picture
|
NADH-Q oxidoreductase is getting 2 electrons from NADH.
E's go to FMN make FMNH2 which gives 2 e's to Fe-S complexes which pull 2 H from matrix to make QH2. 4 H's get pumped out. |
|
|
how does succinate dehydrogenase get FADH2 electrons to Q?
|
Complex II is an integral inner mitochondrial membrane member.
FADH2 never leaves in TCA cycle just gives its electrons to the Fe-sulfur centers in Q |
|
|
How many protons does Q-cytochrome oxidoreductase pump?
|
2 H's from matrix to cytoplasm
transfers electrons from QH2 to cyt c |
|
|
what are cytochromes?
|
electron transfering protein that has a heme prosthetic group.
|
|
|
Name the hemes in cytochrome b.
|
heme bL
(L=lower affinity for electrons) heme bH (H=higher affinity for electrons) |
|
|
name theheme in cytochrome c1.
|
heme c
|
|
|
How many protons get pumped out by Qcytochrome c oxidoreductase?
|
2 H's
|
|
|
What is the Rieske center?
|
2Fe-2S center which has its iron coordinated with His residues in complex III
This coordination raises the reduction potential so it easily accepts electrons from QH2 |
|
|
What is the Q cycle?
|
4 H's released to cytoplasmic side
2 H's drawn up from matrix QH2 is recycled and this is the way we get from a 2 electron carrier (QH2) to a 1 electron carrier (Cyt c) |
|
|
How many electrons do you need to have in complex IV before you can make water?
|
4 electrons
|
|
|
Describe complex IV (what's its name again?)
|
cytochrome c oxidase
has 2 heme A groups 3 Cu ions (2 centers A & B) |
|
|
What in complex IV accepts the electrons initially from cytochrome c?
How does the other Cu center differ? |
Cu A/ Cu A center (cys connected)
CuB has 3 His residues and vascilates btwn Cu+ and Cu2+ to shuttle electrons |
|
|
How does heme A in complex IV differ from the heme in complex III?
|
not covalently attached to the protein
|
|
|
Describe the path of the electron in complex IV?
|
Cu A/ Cu A center
Heme a and heme a3 CuB O2 |
|
|
Describe what happens to electrons in complex IV in order to reduce o2.
|
2 Cyt c's dock---->transfer e's to Cu A/ Cu A--> heme a--->
Fe/heme a3----> Cu B First, CuB is reduced with first electron and then Fe/hemeA3 is reduced with second electron peroxide bridge forms between O's on Fe/hemeA3 and CuB 2 more cyt c drop off their e's and once they get to the Fe/hemeA3 --CuB spot they break the peroxide bridge and 2 H+'s come in Then finally, 2 more protons in and 2 waters leave!! |
|
|
How many chemical protons and how many pumped protons are taken from matrix and put into cytoplasm side?
How do the pumped protons get pumped? |
4 chemical, reacting with the reduction of Oxygen to water and 4 pumped protons
conformational changes make it so the protons can enter from matrix but only exit to cytoplasm |
|
|
What is ROS?
|
Reactive Oxygen Species
superoxide ion formed by partial reduction of O2 |
|
|
What is the pH difference between the cytoplasmic side versus matrix side?
|
1.4 lower pH outside (means more H+ is outside)
|
|
|
what are the 2 components of the proton motive force?
|
chemical gradiant represented by pH (delta pH)
charge gradient represented by delta Psi |
|
|
What is the free energy that corresponds with the 0.14 v difference in the membrane potential?
|
5.2 kcal/mol
|
|
|
Describe ATP synthase.
|
F1 sphere on matrix side is catalytic region (has the a3B3ydE 5 polypeptide thingy
a and B guys are in a hexameric ring and have Ploop NTPase that bind to nucleotides B subunits are the direct catalysts |
|
|
how do the B subunit and y subunit of ATP synthase interact?
|
different y face for each B subunit
|
|
|
Describe the Fo subunit of ATP synthase.
|
in inner mitochondrial membrane
has proton channel which is made up of 10-14 c subunits this is the c ring Top of "arm" binds to outside of ring |
|
|
What is the b2 and d subunits of ATP synthase?
|
b2=long part of outside arm
d= little subunit attached to the F1 subunit |
|
|
Does ATP form in the absence of a proton motive force at the catalytic site?
|
Yes. thing is ATP doesn't leave without the proton motive force.
|
|
|
Describe how the Fo subunit structure allows for the flow of protons.
|
subunit a has 2 hydrophilic half channels (each half-channel interacts with a differnt c subunit)
The c unit has an AsP 61 which grabs a H+ from a's cytoplasmic half channel, rotates and releases H+ to a matrix half channel. |
|
|
How does the unequivalent interaction of the B subunits with the y subunit contribute to binding chain mechanism?
|
Makes one B L-oose
Makes one B T-ight Makes one B O-pen As the y subunit spins around these B's it changes their conformation to all these different forms to bind (L), convert (T) and release (O). |
|
|
What is the role of the ADP-ATP translocase?
|
A protein that moves the ATP and ADP in and out of the inner membrane (remember they do not diffuse--membrane not permeable)
|
|
|
Describe how ATP-ADP translocase works.
|
ADP from cytoplasm binds to the protein causing an eversion where the protein flips over to face the matrix side where it releases ADP and picks up an ATP and then re-flips back to the cytoplasm side
No Mg2+ needed. membrane potential is positive so it favors the negative ATP going to the cytoplasm side |
|
|
What are the two transporters that work together to get ADP and ATP and H's in and out of membrane?
|
ATP-ADP translocase
phosphate carrier these 2 carriers combined with ATP synthase makes the ATP synthasome |
|
|
What does phosphate carrier do?
|
exchanges H2PO4- for OH-
this is basically the way ATP-ADP translocase "pays" for transporting ATP and ADP across the membrane costs 1 H+ |
|
|
How many H+'s do we get pumped for each complex in electron transport chain?
|
Complex 1 (NADH Q oxidoreductase)
4 H+'s Complex III (Q-cytochrome c oxidoreductase) 4 H+'s Complex IV (cytochrome c oxidase) 4 H+ |
|
|
How many ATP are generated per glucose molecule oxidized to CO2?
|
~30
26 are from oxidative phosphorylation NADH's 2e give 2.5 ATP FADH2's 2e give 1.5 ATP |
|
|
How many H+'s must flow through the ATP synthase to make one ATP?
|
3
|
|
|
What does low ADP indicate?
|
resting muscle. no need to synthesize ATP
slows down citric acid cycle, and thus not as much NADH and FADH2 are put into electron transport chain and thus no ATP is made |
|
|
What do rotenone and amytal block?
|
electron transfer in NADH-Q oxidoreductase (complex I)
prevent utilizing NADH succinate oxidation still goes tho |
|
|
what does antimycin A block?
|
electron transfer from cytochrome bH in Q-cytochrome c oxidoreductase (complex III)
|
|
|
What does cyanide (CN-) and azide (N3-) and CO block?
|
electron flow in cytochrome c oxidase (complex IV)
CN- and N3- fuck with ferric heme a3 CO fucks with ferrous form |
|
|
Explain what CO and CN- and N3- do to block electrons and where they do it?
|
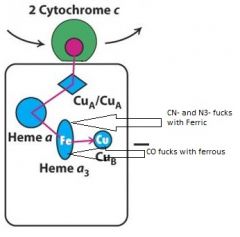
Complex IV--Cytochrome C Oxidase
|
|
|
Explain what blocks electron transport and where in electron transport chain
|
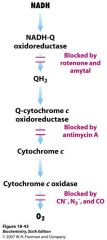
|
|
|
What inhibits H+ influx thru ATP synthase?
|
Oligomycin and DCCD
|
|
|
What does 2, 4-dinitrophenyl (DNP) do as inhibitory?
|
uncouples electron transport chain so that NADH is oxidized but no ATP is produced
|
|
|
What does bongkrekic acid do to inhibit ATP production?
|
mold (like bong water--yuck) that inhibits ATP-ADP translocase
binds where the nucleoside binds and ceases oxidative phosphorylation |

