![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
27 Cards in this Set
- Front
- Back
|
Outline how nitric oxide causes damage to bacteria |
NO can diffuse across the bacterial membrane and react with haem groups, thiols and destroy iron sulphur clusters NO can damage respiratory cytochromes which causes respiratory inhibition NO can react with superoxide produced by NADPH oxidase to form peroxynitrite which can nitrate protein residues |
|
|
When does bacteria encounter NO? |
Dietary nitrites react with stomach acids which causes the formation of nitric oxide Gut bacteria respire anaerobically, nitric oxide is an intermediate of anaerobic respiration. Nitrate/nitrite is used as an electron acceptor which can get reduced to nitric oxide Commensal bacteria produce NO iNOS produce NO during infections eNOS |
|
|
How do most nitric oxide synthases work?
|
Arginine --> Hydroxyarginine --> Citrulline + nitric oxide |
|
|
Describe the structure of LPS |
A core which is connected to an O-specific chain and Lipid A (two glucosamine residues bound to six fatty acids) |
|
|
How does the body detect gram negative bacteria? |
Using its O-specific chain |
|
|
Draw a diagram of inducible nitric oxide synthase (iNOS) |
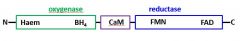
|
|
|
Draw a diagram demonstrating how NO is produced by iNOS
|
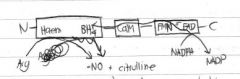
|
|
|
True or false? CaM is activated by Ca2+
|
No, it's calcium insensitive but other isoforms of NOS is activated by Ca2+ |
|
|
What makes CaM calcium insensitive?
|
CaM is covalently bond between calmodulin and calcium |
|
|
In other NOS isoforms, what does NO do? |
Nitric oxide stimulates guanylate cyclase which increases cGMP levels. This stops calcium entry into the cell which results in diminished eNOS dependent nitric oxide production |
|
|
What adaptations does E.coli have which helps it to resist nitric oxide? |
1. Flavohaemoglobin hmp - converts nitric oxide to nitrate (aerobic) and into nitrous oxide (anaerobic)
2. Flavorubredoxin reductase NorVW - nitric oxide converted to nitrous oxide during anaerobic conditions 3. Cytochrome nitrite reductase NrfA - NO reduced 4. Diiron protein YtfE - repairs damaged cytochromes caused by NO damage 5. Cytochrome bd1 - Is a NO-tolerant respiratory oxidase which helps to carry on aerobic respiration |
|
|
What is cytochrome bd-1?
|
It's a NO-tolerant final electron acceptor which reduces oxygen into water. Transfers 2H+ from QH2 into the periplasm which creates a proton gradient which ATP synthase uses to generate ATP |
|
|
What does flavohaemoglobin consist of?
|
FAD-binding domain, NAD-binding domain and a globin |
|
|
How does cytochrome bd-1 help against NO? |
Normally, NO damages respiratory cytochromes which means it can't generate ATP. Cytochrome bc-1 is NO-tolerant meaning that NO doesn't affect it, meaning that it can still produce ATP |
|
|
Outline the reaction for flavohaemoglobin under aerobic and anaerobic conditions |

|
|
|
What catalyses the flavohaemoglobin hmp reaction?
|
The haem cofactor on the flavohaemoglobin hmp |
|
|
What happens to the haem cofactor during the reaction? |
The reaction converts the haem cofactor from the ferrous (Fe2+) to the ferric (Fe3+) form. This is done by oxidising an NAD in the NAD binding site. An electron is donated and moved to the globin area through the FAD co-factor. |
|
|
Outline the two ways hmp converts NO to nitrate |
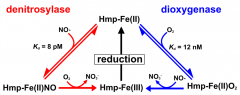
|
|
|
Explain the two ways hmp converts NO to nitrate |
In the early phases of an infection, when lots of oxygen is present and very little NO, they dioxygenase reaction is used. This is when oxygen binds first to convert Hmp-Fe(II) to Hmp-Fe(II)O2, using NO and converting it to NO3-. This produces Hmp-Fe(III) which is then reduced back to Hmp-Fe(II) In the late phases of infection, when lots of NO is present produced by immune cells, the dinitrosylase reaction is taken. This is in which NO binds to Hmp-Fe(II) which turns into HMP-Fe(II)NO. Oxygen is then used to convert the NO to NO3- which produces Hmp-Fe(III), which in turn is reduced back to Hmp-Fe(II). |
|
|
Name two ways hmp is regulated under aerobic conditions |
NsrR AND MetR |
|
|
How is hmp regulated under anaerobic conditions? |
FNR |
|
|
Explain how hmp is regulated under aerobic conditions? |
NsrR -> normally represses hmp transcription, in the absence of NO. When NO is present, the iron-sulphur clusters in NsrR is nitrosylated which causes a loss of DNA binding, damaging NsrR so it can no longer regulate the transcription of hmp, causing hmp to be expressed MetR -> MetR activates hmp transcription. MetR is regulated by homocysteine. When NO is present, homocysteine is damaged and reduced meaning MetR is regulated less. This leads to the transcription of more Hmp genes. |
|
|
Explain how hmp is regulated under anaerobic conditions |
FNR -> NO reacts with the iron-sulphur clusters of FNR. The iron-sulphur centres usualy repress the hmp gene. When NO is present, it damages iron-sulphur clusters which deactivates FNR. This leads to hmp no longer being repressed. |
|
|
Which type of bacteria produce nitric oxide? |
Gram-positive bacteria producing NO are called bNOS |
|
|
What do bNOS lack? |
A reductase domain |
|
|
How is NO produced to give bacteria an advantage? |
It gives bacteria resistance to a variety of antibiotics |
|
|
How are NO-induced advantages achieved? |
1. Chemically altering NO to less toxic compounds 2. Getting rid of oxidative stress imposed by many antibiotics 3. NO suppresses the antibiotic-mediated formation of reactive oxygen species. |

