![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
68 Cards in this Set
- Front
- Back
|
What is an Atom? |
A very small particle that makes up all matter |
|
|
What is an Element? |
A pure chemical substance consisting of one type of atom |
|
|
What is the Mass number? |
Number of protons plus neutrons in the nucleus |
|
|
What is the Atomic number? |
Number of protons in the nucleus |
|
|
what is the Atomic nucleus? |
- Nucleus is at the centre of atom. - contains neutrons and protons - nearly all the atom's mass is in the nucleus |
|
|
What are Protons? |
- heavy - positive charge - in nucleus - tiny but heavy particles found in nucleus of an atom
|
|
|
What are Electrons? |
- light - negative charge - around nucleus - a very light negatively charged particle inside an atom. - electrons move around the central new kris of an atom in different orbits or shells |
|
|
What are Neutrons? |
- heavy - no charge - in the nucleus - tiny but heavy particle found in the nucleus of an atom |
|
|
What is the Atomic symbol? |

Back (Definition) |
|
|
What is Colour? (non metals) |
Many different colours |
|
|
What is Lustre? (metals) |
Bright and shiny when freshly cut |
|
|
What is Electron configuration? |

Back (Definition) |
|
|
What is Malleability? (metals) |
Malleable (easy to bend into shapes) |
|
|
What is Malleability? (non metals) |
Brittle of solid |
|
|
What is Conductivity? (metals) |
Good conductors of heat and electricity |
|
|
What is Conductivity? (non metals) |
Poor conductors of heat and electricity |
|
|
What are the Melting and boiling points for metals? |
Usually very high |
|
|
What are the Melting and boiling points for non metals? |
Usually low |
|
|
What is an ion? |
An atom that has lost or gained one or more electrons. It does not have the same number of protons and electrons. |
|
|
What is a cation? |
A positive ion. E.g. Metal ion that has lost an electron |
|
|
What is an alpha particle? |
A helium nucleus - two protons and two neutrons |
|
|
What is the alpha particle symbol? |
As in table |
|
|
What is Beta particle? |
A high speed electron emitted from the nucleus |
|
|
What is a Compound? |
- A compound is a chemical substance consisting of two or more different chemical elements. - Chemical compounds have a fixed ratio of atoms. |
|
|
What is the alpha particle symbol? |
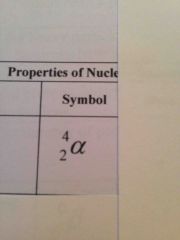
Back (Definition) |
|
|
What is the beta particle mass? |
Zero |
|
|
What is the beta particle charge? |
-1 |
|
|
What is the beta particle speed? |
Fast |
|
|
What is the beta particle Penetrating power? |
Medium |
|
|
What is the better particle symbol? |
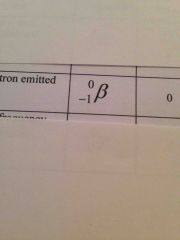
Back (Definition) |
|
|
What is the gamma ray charge |
0 |
|
|
What is the gamma Ray symbol? |
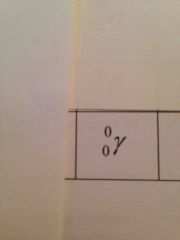
What is the gamma Ray symbol? |
|
|
What is the gamma ray penetrating power? |
High |
|
|
What are Isotopes? |
- Isotopes are atoms of the same element that contains a different number of neutrons in their nuclei. - isotopes have the same atomic number but different mass number. |
|
|
What is Nuclear radiation? |
Radiation that is emitted from the nucleus of an atom |
|
|
What is radioactive? |
An atom that emits nuclear radiation |
|
|
What is half-life? |
The time taken for half of an amount of radioactive material to decay |
|
|
What is light? |
A kind of energy |
|
|
How is light emitted? |
Hot gases, liquids and solids |
|
|
Can objects emit their own light? |
Yes |
|
|
What happens when light hits a smooth shiny surface? (Mirror) |
It bounces back |
|
|
What is a lense? |
A lense is a curved piece of glass that refracts light |
|
|
What is a concave lense? |
The lense is thinner in the middle than at the outside. |
|
|
What is the smallest unit of matter? |
Atoms |
|
|
What are the three types of sub atomic particles? |
Protons, neutrons and electrons |
|
|
What is the charge of chlorine atom? |
Negative |
|
|
Which sub-atomic particle has a negative charge? |
Electrons |
|
|
What is an element mass number? |
Number of protons plus neutrons |
|
|
Describe the structure of an atom? |
Centre is nucleus outside is protons |
|
|
How many electrons will fit in each of the first four electron shells? |
Eight |
|
|
Explain why atoms tend to form ions? |
Two electrons are added to make a full shell. |
|
|
What is the atomic number of an element? |
Number of protons in an atom |
|
|
Who arranged the periodic table in its current form? |
Dmitri Mendeleev |
|
|
How many neutrons does an atom of fluorine have if its atomic number is nine and its nest number is 19? |
Nine |
|
|
What is the Valence shell? |
The outer shell of electrons |
|
|
How would you calculate the number of neutrons in the nucleus of an atom of an element? |
If you know that the mass number and atomic number of the atom, the number of neutrons is just the difference between them. |
|
|
What is matter? |
Physical substance is general, as distinct from mind as spirit. |
|
|
What are Noble gases? |
Group VIII elements that have a full outer shell of electrons |
|
|
What is the Octet rule? |
The outermost shell had a maximum of 8 electrons |
|
|
What are the states for metals? |
All solids except Mercury |
|
|
What are the states for Non metals? |
Solids, liquids and gases |
|
|
What is refraction? |
Describes how light leads when it enters a medium that is more or less dense. |
|
|
What is refraction? |
Describes how light leads when it enters a medium that is more or less dense. |
|
|
What is a lense? |
A curved piece of glass that refracts light? |
|
|
What is refraction? |
Describes how light leads when it enters a medium that is more or less dense. |
|
|
What is a lense? |
A curved piece of glass that refracts light? |
|
|
How can you test how strong a lens is? |
By shining a beam of parallel light through it. |
|
|
What is the difference between a strong lens and a weak lens? |
Strong lens is thick in the middle and weak lens is thin in the middle |

