![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
41 Cards in this Set
- Front
- Back
|
Weathering to Form Sediment
|
1. Weathering Changes
2. Mechanical Weathering 3. Chemical Weathering |
|
|
Weathering Changes in Conditions
|
1. Different conditions at earth surface than at depth
A. Low temperature B. Low pressure C. Presence of significant organic activity in recent geologic past |
|
|
Weathering Changes in Abundance: Minerals that form
|
1. Changes % of minerals in sediment compared to % in rock
A. Average GRANITE (CONTINENTAL CRUST) - 80% feldspar - 15% QUARTZ - 10% hornblende, mica, and others B. Average SANDSTONE (detrital rock) - 85% QUARTZ - 15% chert, feldspars, rock fragments, and others 2. Destroys labile minerals 3. Makes new stabile minerals A. Most abundant sedimentary rock is Mudrock with abundant clay minerals |
|
|
Weathering Changes in Abundance
|
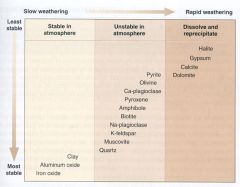
|
|
|
Minerals that Form in Soils
|

1. Not common in Granite
2. Very common in Soils 3. Some very common in A. Sedimentary Rocks - Clay (hydrous Al silicates) - Aluminum oxides - Iron oxides |
|
|
Minerals that MAY Persist in Soils
|
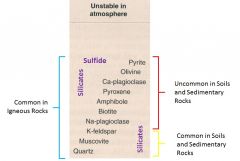
1.Uncommon in Soils and Sedimentary rocks
A. Sulfide - Pyrite B. Silicates - Olivine - Ca-plag - Pyroxene - Amphibole - Biotite - Na-plag 2. Common in Soils and Sedimentary Rocks A. Silicates - K-spar - Muscovite - Quartz |
|
|
Non-clastic
|
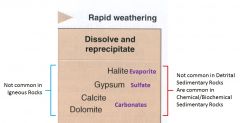
1. Not common in detrital sedimentary rocks, are common in Chemical or Biochemical sedimentary rocks
A. Carbonates - Dolomite - Calcite B. Sulfate - Gypsum C. Evaporite - Halite |
|
|
Weathering Processes
|
1. Physical
2. Chemical |
|
|
Physical and Chemical Weathering along Joints and Bedding Planes
|
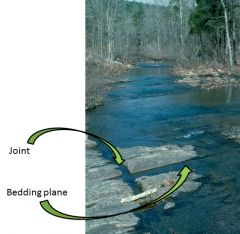
|
|
|
Physical Weathering
|
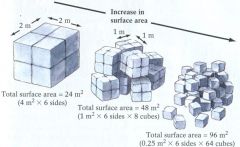
1. Reduces grain size
2. Does NOT change chemistry or mineralogy |
|
|
Three Processes
|
1. Expansion/Contraction
A. Insolation - Crystals expand with heat B. Hydration/dehydration - Some crystals expand with water C. Freeze Thaw - Ice increases 9-10% by volume compared to water 2. Stress release (unloading) 3. Organic Activity |
|
|
Thermal Expansion and Contraction:Fire-cracked Rock
|

|
|
|
Salt Crystal Hydration
|

|
|
|
Salt Hydration??
|

|
|
|
Freeze/Thaw
|
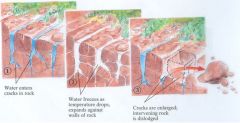
|
|
|
Frost Wedging forms Talus
|

|
|
|
Stress Release - Exfoliation
|
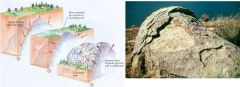
|
|
|
Exfoliation Dome: Independence Rock
|

|
|
|
Organic Activity - Root Wedging
|

|
|
|
Properties of Water that Contribute to CHEMICAL WEATHERING
|
1. Polar
2. Dissociates 3. Surface tension and capillarity |
|
|
Polarity
|
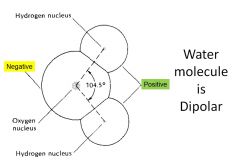
|
|
|
Water Dissociates
|
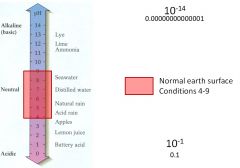
1. pH is the hydrogen ion concentration
2. pH neutral = 7 A. H+1 and OH-1 = 10-7 at 25o C 3. pH acid = 1-6 4. pH alkaline 8-14 5. Normal range = 4-9 |
|
|
Acidity = abundance of H ion
pH 1-14 |
|
|
|
Water dissociates to form acids in soils
|
1. H2O + CO2 ---> H2CO3
2. H2CO3 ---> H+ + HCO3- 3. CO2 is present as a soil gas derived from plant respiration 4. H2CO3 is carbonic acid 5. HCO3- is bicarbonate |
|
|
Processes of Chemical Weathering
|
1. Dissolution
2. Hydrolysis – solid products 3. Hydration – solid products 4. Oxidation – solid products |
|
|
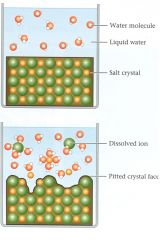
Dissolution by dipolar H2O
|
|
|
|
Dissolution
|

1. NaCl + H2O ---> Na+1 + Cl -1
2. All products are in solution |
|
|
Hydrolysis – new mineral
|
1. K Feldspar + H2O ---> Clay mineral + K +1
Silicate + water ---> hydrous aluminum silicate (solid) + K +1 (solution) |
|
|
Hydration – “same” mineral
|
1. Anhydrite + H2O ---> Gypsum
2. CaSO4 ---> CaSO4 . H2O |
|
|
Oxidation – new mineral
|

1. 2Fe+2O-2 + O2 ---> Fe2+3O3-2
Magnetite ---> Hematite 2. 2Fe(+2)S2(-4) + O2 ---> Fe2(+3)O3(+2) + 2S(-4) Pyrite ---> Hematite |
|
|
Oxidation/Reduction: Redox in the Weathering Environment
|
1. OXIDATION
A. Atom or ion loses electrons (becomes more positive) B. Best oxidizing agent is atmospheric oxygen, O2 C. Normally occurs above the water table where water is present and O dissolved in water is abundant. D. Fe++ ---> Fe +++ E.Metallic ions (Fe, Mn) are usually immobile in oxidizing environments |
|
|
Oxidation/Reduction: Redox in the Weathering Environment
|
1. REDUCTION occurs when an ion in a mineral structure gains an electron.
2. This normally occurs below the water table where water is present but dissolved O in the water is absent. 3. Fe+++ ---> Fe ++ 4. Metallic ions (Fe, Mn) are usually more mobile in reducing environments |
|
|
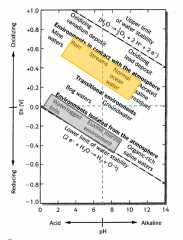
Eh
|
1. The tendency for oxidation or reduction to occur is the REDOX POTENTIAL
A. Measured in millivolts (electricity) B. Positive = oxidation C. Negative = reduction |
|
|
Eh/pH Conditions of Water
|
|
|
|
Ion Exchange
|
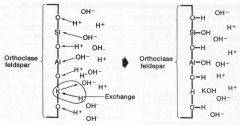
1. Substitution of an ion in a mineral structure for an ion in solution
2. Cation Exchange Capacity (CEC) in milliequivalents per 100 g of clay 3. Important for clay mineral weathering 4. Ca++, Na+, Mg++, K+, and Fe++, are mobile V. Si+++, Ti++++, Fe+++, Al+++ are immobile |
|
|
Ion Mobility: Processes
|
1. Temperature
2. pH 3. Eh 4. Through Flow |
|
|
Ion mobility: Chart
|
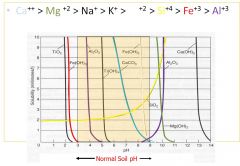
Ca++ > Mg++ > Na+ > K+ > Fe++ > Si++++ > Fe+++ > Al+++
|
|
|
Silica Solubility due to pH depends on crystallinity
|
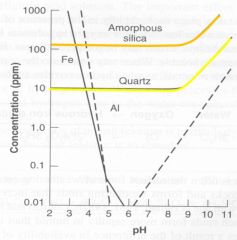
|
|
|
Weathering Products
|
1. Silicates
A. Clay minerals – hydrous Al silicates formed by hydrolysis B. Quartz – concentrated because immobile 2. Oxides A. Fe and Mn – concentrated because immobile B. Al – concentrated because immobile |
|
|
Weathering Susceptibility
|
1. Bonding
2. Rate 3. Cation mobility 4. Mineral mobility 5. Grain size 6. Voids 7. Si-O bonds become stronger as more O ions shared between tetrahedra 8. Other cation-O bonds weaker |
|
|
Organic Preservation
|
1. No free oxygen
A. Stagnant fresh water - Swamps - Bogs B. Below groundwater table C. Stagnant sea water - Euxinic marine environment - Organic-rich saline water |

