![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
What are biological molecules |
molecules made and used by living organisms e.g. Carbohydrates, Proteins, Lipids, DNA, ATP, Water, Inorganic Ions |
|
|
What are the functions of carbohydrates |
energy source (glucose in respiration) energy store (starch in plants, glycogen in animals) structure (cellulose in cell wall of plants) |
|
|
What are the building blocks for carbohydrates called |
monosaccharides |
|
|
Example of monosaccharides |
- glucose (alpha and beta), - galactose, - fructose |
|
|
Formula for monosaccharides |
C6H12O6 (isomers = same formula but different arrangement) |
|
|
Difference between alpha and beta glucose |
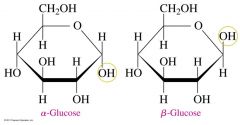
on Carbon 1, alpha glucose has a OH group on the bottom and beta glucose has a OH group on the top. |
|
|
Bond in carbohydrate |
glycosidic bond |
|
|
How are monosaccharides joined together |
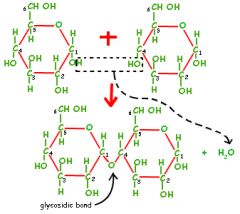
condensation reaction (removing water) – between 2 OH groups. |
|
|
Example of disaccharides |
glucose + glucose = maltose glucose + galactose = lactose glucose + fructose = sucrose |
|
|
Formula for disaccharides |
C12H22O11 |
|
|
How are polymers separated |
hydrolysis (add water) |
|
|
What is a polysaccharide |
Many monosacharrides joined by condensation reaction/glycosidic bonds. |
|
|
Example of polysaccharides |
1. Amylose (long chain of alpha glucose) which makes starch/glycogen. 2. Cellulose (long chain of beta glucose) which makes cell wall in plants. |
|
|
What are Polysaccharides |
carbohydrates made of a long chain of monosaccharides joined by condensation reaction/glycosidic bonds. 3 examples: Starch, Glycogen, Cellulose. |
|
|
Properties of Starch and Glycogen as energy stores |
Insoluble = do not affect water potential of the cell, do not diffuse out of the cell Coiled/Branched = compact, more can fit into a cell Branched/Chained = glucose removed from the end |
|
|
Structure of Cellulose |
1. β-glucose arranged in a straight chain (each alternative β-glucose is rotated 180 degrees). 2. Many cellulose chains are cross linked by hydrogen bonds to form microfibrils. 3. Many microfibrils are cross linked to form marcrofibrils. Creates a strong material. |
|
|
Test for starch |
add iodine, turns blue/black |
|
|
Test for reducing sugar |
heat equal ammounts of sample and Benadict's regent, turns brick red. |
|
|
Test for non-reducing sugar |
1. Test for reducing sugars 2. Take a new sample and add dilute hydrochloric acid (hydrolyses glycosidic bond) and heat gently. 3. Slowly Add sodium hydrogencarbonate (neutralises solution) until solution is alkaline. |
|
|
What are 2 types of proteins |
Globular and Fibrous |
|
|
What are globular proteins |
Soluble proteins with a specific 3D shape e.g. enzymes, hormones, antibodies, haemoglobin. |
|
|
What are fibrous proteins |
strong/insoluble/inflexible material e.g. collagen and keratin |
|
|
What are the building blocks for proteins |
Amino acids |
|
|
Structure of amino acid |
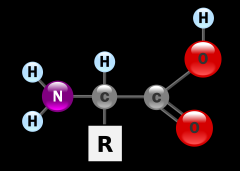
central carbon, carboxyl group to the right (COOH), amine group to the left (NH2), hydrogen above and R group below. |
|
|
How do amino acids differ |
have different R groups e.g. glycine has a hydrogen in its R group – simplest amino acid |
|
|
How are amino acids joined together |
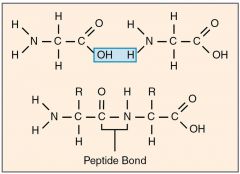
By condensation reaction between the carboxyl group of one and amine group of another, leaves a bond between carbon & nitrogen (called a peptide bond) forming a dipeptide. |
|
|
Define primary, secondary, tertiary, quaternary structure |
Primary = sequence of AA, polypeptide chain (held by peptide bonds) Secondary = the primary structure (polypeptide chain) coils to form a helix, held by hydrogen bonds Tertiary = secondary structure folds again to form final 3d shape, held together by hydrogen/ionic/disulfide bonds Quaternary = made of more then one polypeptide chain |
|
|
Examples of quaternary structure proteins |
collagen (3 chains), antibodies (3 chains), haemoglobin (4 chains) |
|
|
Structure of collagen |
strong material, used to build tendons/ligaments/connective tissues.
1. Primary structure mainly made up of glycine. 2. Secondary structure forms a tight coil (not much branching due to glycine) 3. Tertiary structure coils again 4. Quaternary structure made from 3 tertiary structures wrapped around each other like rope. |
|
|
Test for protein |
1. Mix equal volumes of sodium hydroxide solution and the sample. 2. Add a few drops of dilute Copper(II) Sulphate solution. 3. Will turn purple in the presncence of proteins. |
|
|
What is an enzyme |
A biological catalyst that lowers activation energy required for a reaction top take place. |
|
|
What makes an enzyme specific |
It has a specific active site shape, only complementary substrates can bind to the active site to form enzyme-substrate complexes. |
|
|
Lock and Key Model vs Induced Fit Model |
LK = active site shape is rigid, only exactly complementary substrates can bind to form ES complexes. IF = active site changes shape, the substrate binds to the active site – the active site changes shape so the substrate fits exactly forming an ES complex. |
|
|
Affect of substrate concentration on enzyme activity |
1. Increase substrate concentration.
2. Increases chance of successful collisions. 3. Increase chance of forming an ES complex. 4. Increase rate of reaction. |
|
|
Affect of enzyme concentration on enzyme activity |
1. Increase enzyme concentration,
2. Increases chance of successful collisions, 3. Increase chance of forming an ES complex, 4. Increase rate of reaction |
|
|
Affect of temperature on enzyme activity |
1. As temperature increases the kinetic energy increases which causes the molecules to move faster.
2. There are more collisions occer which increases the chance of forming an ES complex. 4. So the rate of reaction increases until the optimum is reached. 5. Hydrogen and ionic bonds in tertiary structure break causing the structure of the molecule to change. So the substrate is no longer complimentary to the active site. 6. Less ES complexes form, reducing the rate of reaction. |
|
|
Affect of pH on enzyme activity |
If change pH away from optimum, bonds in tertiary structure break, lose active site shape, no longer form ES complex, the enzyme becomes denatured. |
|
|
Competitive vs Non-Competitive Inhibitors |
Competitive = a substance with a similar shape to the substrate and a complementary shape to the enzyme's active site, binds to the active site, blocking it, preventing ES complexes from forming. Non-Competitive = a substance that binds to another site on the enzyme other then the active site, causes the active site to change shape, so less ES complexes can form. |
|
|
What are the 3 types of Lipids |
Triglycerides (fat for energy store, insulation, protection of organs). Phospholipids (to make membranes). Cholesterol (for membrane stability and make hormones). |
|
|
Structure of triglyceride |
Made of 1 glycerol and 3 fatty acids which are joined by condensation reaction. there are 2 types of triglycerides: saturated fat and unsaturated fat |
|
|
Saturated vs Unsaturated Fat |
Saturated = has no carbon double bonds in the R group of the fatty acid Unsaturated = has carbon double bonds in the R group of the fatty acid |
|
|
Structure of phospholipid |
made of 1 glycerol, 2 fatty acids and 1 phosphate phosphate forms a hydrophillic head, fatty acids form hydrophobic tails forms a phospholipid bilayer, basic structure of membranes |

