![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
Scientific method |
Something scientists use to solve a problem |
|
|
Hypothesis |
An educated guess |
|
|
Controlled experiment |
Changes 1 variable to see the effect |
|
|
Scientific method order |
Question data hypothesis experiment conclusion reporting |
|
|
Define control |
The variable that does not change/ does not include the independent variable |
|
|
Define independent variable |
The variable that does change |
|
|
Dependent variable |
The result of the independent and control variables in comparison |
|
|
Define qualitative |
A description such as wet, hot, or shiny |
|
|
Define Quantitive |
The amount of something like 30 km or a ph of 5.2 |
|
|
Define conclusion |
Describing the results of the experiment |
|
|
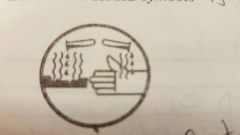
Corrosive material |
|
|
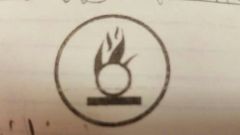
Oxidizing material |
|

|
Poisonous and infectious material causing other toxic effects |
|
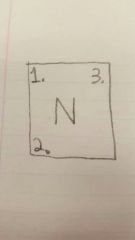
1 |
Atomic Number |
|
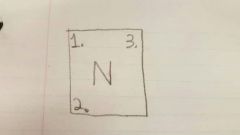
1 |
Atomic Number |
|
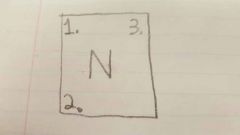
2 |
Atomic Mass/ Mass # |
|
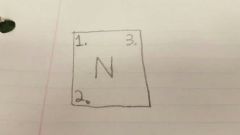
3 |
Ion charge |
|
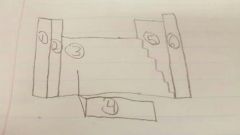
|
1: alkali earth metals 2: alkaline earth metals 3: transition metals 4: lanthanides and actinides 5: non metals 6: noble gasses
|
|
|
Define Element |
Pure substance made of one type of atom |
|
|
Define Atom |
Smallest piece of an element because it has the properties |
|
|
Define Subatomic Particles |
The particles that make up an atom |
|
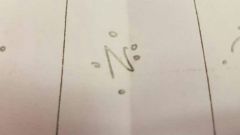
What do the dots represent |
Number of electrons in outer shell |
|
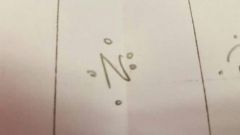
What does the letter represent |
The element. |
|

How many electrons in its valence shell? |
6 |
|

What are the grey dots |
Electrons |
|

What are the yellow dots |
Neutrons |
|

What are the purple dots |
Protons |
|
|
What is an electrons charge |
Negative |
|
|
What is a protons charge |
Positive |
|
|
What is a neutrons charge |
Neutral |
|
|
Define ionic bond |
A combination of a metal and a polyatomic Ion |

