![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
|
What is an atom? |
Smallest particle of an element that will has properties of that element. |
|
|
Atoms join together to form ___ |
Compounds |
|
|
A chemical change occurs when... |
The arrangement of atoms in a compound changes to form new compounds. |
|
|
Atoms are made up of smaller particles called _____ |
Subatomic particles |
|
|
What is the symbol, electron charge, location in an atom, and relative mass of a proton? |
Symbol = P E. Charge = 1+ Location= Nucleus Relative Mass = 1836 |
|
|
What is the symbol, electron charge, location in an atom, and relative mass of a neutron? |
Symbol = n E. Charge = 0 Location = Nucleus Relative Mass = 1837 |
|
|
What is the symbol, electron charge, location in an atom, and relative mass of an electron? |
Symbol = e E. Charge = 1- Location = Surrounding the nucleus Relative Mass = 1 |
|
|
The nucleus is at the ____ of the ____ |
Centre Atom |
|
|
What is the nucleus composed of? |
Protons and neutrons |
|
|
Electrons exist in the space surrounding the ____ |
Nucleus |
|
|
What is equivalent to the # of Protons? |
# of Electrons in every atom (neutral) |
|
|
What is the nuclear charge equivalent to? |
# of protons |
|
|
What is the atomic # equivalent to? |
# of protons |
|
|
What is the atomic mass equivalent to? |
# of protons + neutrons |
|
|
How do you find neutrons? |
Atomic Mass - Atomic # |
|
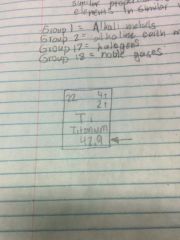
What is it? |
Average Atomic Mass |
|
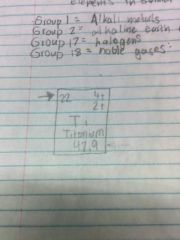
What is it? |
Atomic Number |
|
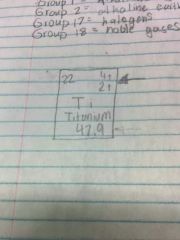
What is it? |
Ion charge |
|
|
What is a Bohr diagram? |
Diagram that shows # of electrons in each shell surrounding the nucleus |
|
|
What is a stable octet? |
8 electrons in the outermost shell |
|
|
What is the valence shell? |
The outermost shells that contains electrons. |
|
|
What are valence electrons? |
Electrons in the valence |
|
|
In a period table, elements are listed in order by their ___ ___ |
Atomic Number |
|
|
Where are the metals in the table? |
Left side of the table (group 3 - 12) |
|
|
Where are the non metals on a table? |
Right side of the table |
|
|
Where is the metalloids on the table? |
Staircase towards the right side |
|
|
Rows of elements are called ___ |
Periods |
|
|
Columns of elements are called |
Groups and families |
|
|
All elements in a period have their ___ in the same general area around their ___ |
Electrons, nucleus |
|
|
Atoms become electrically charged particles called... |
Ions |
|
|
Non metals lose electrons and become positive ions called |
Anions |
|
|
Metals lose electrons and become positive ions called |
Cation |
|
|
Atoms gain/lose electrons in an attempt to.. |
Have the same number of valence electrons |

