![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
81 Cards in this Set
- Front
- Back
|
which 3 factors affect the transport |
(1) The oxygen diffusion gradients (2) The carbon dioxide diffusion gradients (3) Haemoglobin's oxygen dissociation relationship |
|
|
whats the PO2 in the alveoli |
104mm Hg |
|
|
PO2 in the blood in alveolar capillaries |
40 mm Hg |
|
|
since the PO2 in the alveoli is 104 mm Hg and PO2 of blood in alveolar capillaries 40mm Hg |
O2 diffuses into the blood until blood PO2 reaches 104mm Hg |
|
|
whats the PO2 of blood leaving the alveolar capilaries |
104 mmHg |
|
|
whats the PO2 of the blood leaving capillaries when it eaches pulmonary vein |
95 mmgH |
|
|
whats the pO2 of interstitial fluid |
40 mm Hg |
|
|
pO2 of cells |
20 mmgH |
|
|
What's the intracellular pCO2 |
46mmHg |
|
|
interstitial fluid pCO2 |
45mm hg |
|
|
whats the pCO2 of capillaries supplying tissues with blood ? |
40 mmHg |
|
|
pCO2 of alveoli |
40 mmHg |
|
|
in which way does CO2 of the alveoli diffuse and why ? |
from blood into alveoli because pCO2 of deoxygenated blood in Pulmonary artery is 45 mmgh and pCo2 of alveoli as about 40 mmHg. |
|
|
how much of a red blood cell's weight made up of haemoglobin |
1/3 |
|
|
erythrocytes remove blood from where ? |
plasma and allow them to diffuse into blood |
|
|
how lond does erythropoesis take |
4 days |
|
|
where does erythropoesis take place |
bone marrow |
|
|
how is erythropoesis stimulated ? |
Low PO2 stimulates erythropoiesis by increasing the formation of the glycoprotein erythropoietin in the kidneys. Erythropoietin induces the bone marrow to make more erythrocytes. |
|
|
describe the structure of haemoglobin |
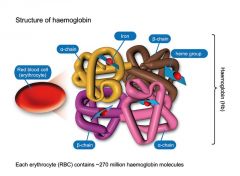
Each haemoglobin molecule is made up of 4 polypeptides, called globins, which each bind one heme (haem) molecule |
|
|
what molecule binds to heme |
1 iron molecule |
|
|
which molecule binds oxygen |
iron |
|
|
how many alpha and beta chains do aldult haemoglobin molecule consist of ? |
• Adult haemoglobin consists of 2 alpha chains and 2 beta chains |
|
|
whats oxygen rich haemoglobin called |
oxyhaemoglobin
(deoxyhaemoglobin when lacks oxygen) |
|
|
the rxn in which O2 binds to iron ions in haemoglobin (Hb) molecules to form oxyhaemoglobin (HbO2 ) is : a) reversible b) irreversible |
b) reversible. |
|
|
how many oxygens does a haemoglobin mol. bind ? |
4
(Each RBC has ~280 million Hb molecules, each binds 4 oxygen molecules) |
|
|
what percentage of O2 is carried by haemoglobin |
97% |
|
|
describe Hb's oxygen dissociation relationship |
-Hb carries 97% of oxygen, 3% is arried in plasma -O2 binds to the Hb in the lungs and dissociates from the haemoglobin into the tissues • The relationship between O2 and Hb is described by the oxygen dissociation curve |
|
|
How does CO poisoning occur |
CO binds strongly to Hb and takes up the place of O2 causing CO poisonig
Hydrogen cyanide (HCN) also binds to Hb |
|
|
what is Oxygen–Haemoglobin Saturation Curve |
Graph relating saturation of Hb to partial pressure of O2 |
|
|
-------------- PO2 pressure results in greater Hb saturation
a. higher b. lower |
Higher |
|
|
why is the Oxygen–Haemoglobin Saturation Curve a curve rather than a straigh line ? |
because Hb changes shape each time a molecule of O2 is bound |
|
|
each time an O2 is bound to Hb it makes it easier for next O2 to bind, why is this an advantage |
• Allows Hb to bind O2 when O2 levels are low |
|
|
whats Hb saturation |
the percentage of heme units in a haemoglobin molecule that contains bound oxygen |
|
|
Environmental Factors Affecting Hb |
PO2 of blood, Blood pH, Temperature, Metabolic activity within RBCs |
|
|
what happens to the The Oxygen–Haemoglobin Saturation Curve when temp rises |
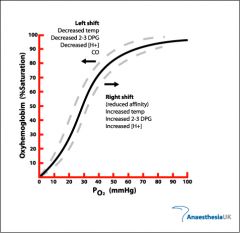
more o2 is released curve shifts right |
|
|
what happens to the The Oxygen–Haemoglobin Saturation Curve when temp drops |
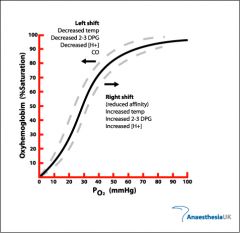
less O2 releases curve shifts left |
|
|
at what PO2 is Hb in erythroytes nearly 100% saturated |
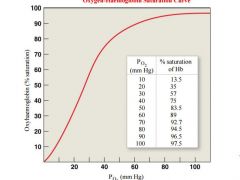
Haemoglobin in the erythrocytes is nearly 100% saturated with oxygen at PO2 of 80 mm Hg and above, thus the blood in the alveolar capillaries is saturated |
|
|
what is the O2 saturation percentage at pO2 of blood capillaries (4o mmhg) |
• The PO2 of blood in the capillaries of the tissues is about 40mm Hg. At PO2 of 40mm Hg, haemoglobin is about 75% saturated, so about 25% of the O2 carried by the erythrocytes can dissociate into the interstitial fluid |
|
|
describe the relationship between pO2 in IF in skeletal muscle and Hb during exercise |
• During exercise, the PO2 of the interstitial fluid in skeletal muscle can drop to 15 mm Hg. At PO2 of 15 mm Hg, haemoglobin gives up 75% of its O2 |
|
|
what is the effect of low ph on Hb O2 binding |
•The ability of haemoglobin to bind O2 at any PO2 declines with decreasing pH • Decreases i |
|
|
what causes the pH to decrease |
increase of H+ ions |
|
|
what happens as PCO2 increases ? |

•As PCO2 increases, the CO2 combines with water to form carbonic acid. Carbonic acid dissociates into H+ ions and bicarbonate ions •The hydrogen ions then bind to the protein part of the haemoglobin molecule causing a conformational change, decreasing the affinity of haemoglobin for O2
|
|
|
whats the bohr effect? |
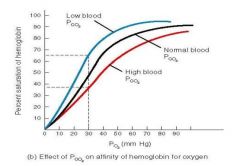
a decrease in pH and an increase in the CO2 concentration mean that a greater PO2 is required to saturate the haemoglobin - the oxygen dissociation curve shifts to the right. |
|
|
effects of pH on oxygen-hb curve |
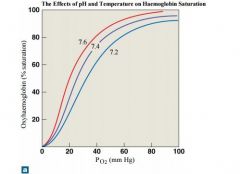
When the pH drops below normal levels, more oxygen is released; the oxygen–haemoglobin saturation curve shifts to the right. When the pH increases, less oxygen is released; the curve shifts to the left. |
|
|
Role of temperature on O2-Hb curve |
• Increases in temperature shift the oxygen dissociation curve to the right, as does the accumulation of acidic by products such as lactic acid • Temperature effects are significant only in active tissues that are generating large amounts of heat, e.g., active skeletal muscles • In heavy exercise, CO2 and lactic acid accumulate in the tissues and the temperature increases, resulting in as much as 85% of the O2 being released from the haemoglobin • However, in the lungs, because of an increased respiration rate, PCO2 in the lungs decreases and the oxygen dissociation curve shifts further towards the left allowing the haemoglobin to saturate more readily |
|
|
effects of low and high temp on o2-Hb curve |
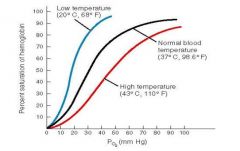
When the temperature rises, more oxygen is released; the oxygen–haemoglobin saturation curve shifts to the right 2,3-bisphosphoglycerate (B |
|
|
2,3-bisphosphoglycerate (BPG) |
• Erythrocytes generate ATP by glycolysis, forming lactic acid and BPG • BPG levels rise when pH increases or when stimulated by certain hormones • BPG directly affects O2 binding and release: It decreases the affinity of haemoglobin for O2 . If BPG levels are too low, haemoglobin will not release O2 • People in high altitudes have high concentrations of BPG in the blood and this is thought to increase oxygen delivery to the tissues at high altitudes • BPG levels in stored blood decrease with tim |
|
|
In what way does BGP directly affect o2 binding and release ? |
It decreases the affinity of haemoglobin for O2 . If BPG levels are too low, haemoglobin will not release O2 |
|
|
• Foetal Haemoglobin structure |
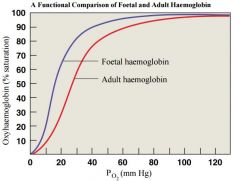
-different from adult - Foetal Hb binds more O2 than adult Hb • This allows foetus to take O2 from maternal blood |
|
|
what 3 ways is CO2 transported in the blood? |
(1) In the form of bicarbonate ions (72%) (2) Combined with blood proteins (20%) – bound to Hb (3) As carbon dioxide dissolved in the plasma (8%) |
|
|
what are blood proteins which bind to CO2 called ? what is formed when they bind to hb? |
• The blood proteins that bind CO2 are called carbamino compounds • CO2 binds to form carbaminohaemoglobin |
|
|
whats the haldane effect |
• The affinity of Hb for CO2 is greater if that Hb molecule has just given up oxygen
(The affinity of Hb for CO2 is greater if that Hb molecule has just given up oxygen) |
|
|
Exchange of O2 and CO2 in pulmonary and systemic capillaries |
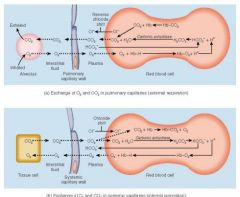
|
|
|
describe the transportation of CO2 as bicarb ions (chloride shift) |
• CO2 diffuses into the erythrocytes and reacts with water to form carbonic acid • This then dissociates into hydrogen ions and bicarbonate ions • The negative bicarbonate ions diffuse out of the erythrocytes into the blood plasma • As a result of this movement out of bicarbonate ions, negative chloride ions move into the erythrocytes from the blood plasma - this maintains the electrical balance between the inside and the outside of the cells • This exchange of negative chloride ions for negative bicarbonate ions is called the chloride shift |
|
|
whats the chloride shift in general terms |
exchange of negative chloride ions for negative bicarbonate |
|
|
H+ ions |
• Hydrogen ions also form from the carbonic acid. Most of these hydrogen ions bind to Hb (reducing it). If hydrogen ions were to diffuse out of the erythrocytes, the pH of the plasma would drop • When the deoxygenated blood reaches the alveoli, the CO2 in solution in the blood diffuses into the alveoli and CO2 bound to the blood proteins dissociates and also diffuses into the alveoli • As the PCO2 of the blood decreases, bicarbonate ions diffuse back into the erythrocytes and Hb releases the hydrogen ions • The bicarbonate ions then bind with the hydrogen ions to form carbonic acid. During this process, the chloride ions move out of the erythrocytes • The carbonic acid then dissociates into water and CO2 and the CO2 diffuses towards the alveoli. Associated with this movement of hydrogen ions into the erythrocytes is a slight increase in blood pH and an associated increase in the uptake of oxygen by the Hb |
|
|
pO2 in alveoli and alveolar capillaries, which way does O2 diffuse ? |
alveoli: 104 alveolar capillaries: 40 o2 diffuses from alveoli into capillaries |
|
|
pO2 in interstitial fluid and cells |
interstitial fuild: 40 cells: 20 blood diffuses from IF into cells
|
|
|
pO2 from alveolar capillaries by the time it reaches pulmonary vein |
104mmg pulmonary vein: 95mmhg |
|
|
cells PCO2 |
46mmhg |
|
|
interstitial fluid pco2 |
44mmhg |
|
|
cappilaries supplying tissue PCO2 |
45mmgh |
|
|
what fraction of RBC is Hb |
1/3 |
|
|
lifespan of RBC in men and women |
120 and 110 |
|
|
how long does erythropoesis take ? |
4 days |
|
|
what stimulates erythropoesis |
stimulated by low PO2 in the kindeys which leads to the formation of glycoprotein which stimulates eruthropoesis in the RBM |
|
|
why is the o2-hb graph curve |
hb changes shape each time o2 molecule is bound |
|
|
what is a o2bh curve |
graph relating to saturation of hb to partial pressure of o2 |
|
|
what results in higher hb saturation |
higher po2 |
|
|
what is hb saturation |
% of heme in a hb molecule which contains bound o2 |
|
|
what happens to the curve when PH drops or Temp rise |
more O2 is released curve shifts to the right |
|
|
what happens to the curve as ph drops |
shifts to the right more o2 released |
|
|
what happens when ph rises and temp drops |
less o2 released curve shifts left |
|
|
what happens to O2 Hb binding with increasing pH? |
declines |
|
|
how does increasing pH decrease Hb O2 affinity |
caused by increased pCO2 this causes CO2 to react with water forming carbonic acid which dissociated into h+ and bicarb ions H+ ions increase pH H+ ions bind to protein part of Hb causing conformational change decreasing affinity Hb for O2 |
|
|
what happens to curve as temp increases |
shifts to the right more o2 released |
|
|
in what tissues is the effect of temp significant on the curve |
active tissue that generate large amounts of heat e.g active skeletal muscle |
|
|
describe effect of BPG on Hb and O2 binding affinity |
released in glycolysis with lactic acid BPG rise with temp in pH increase in BGP increases Hb affinity for O2 low levels of BGP cause o2 to stay bound to Hb |
|
|
describe foetal Hb |
binds more o2 than adult allows it to take up o2 from maternal blood |
|
|
haldane effect |
affinity of hb for co2 is greater if hb has just given up o2 |
|
|
chloride shift |
maintains electrolyte balance cl- moves into rbc as HCO3 diffuses out if the rbc |

