![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
|
Electromagnetic radiation
-definition |
-combination of electric and magnetic fields traveling together
|
|
|
Types of Electromagnetic Radiation
|
-Gamma Rays (high frequency)
-X-rays -visible light -microwaves -Radiowaves (low frequency) |
|
|
Types of electromagnetic radiation used for diagnostic imaging
|
-x-rays (radiographs & CT)
-gamma rays (nuclear medicine) |
|
|
Non-ionizing radiation
-defintion |
-low energy radiation that does not result in the removal of electrons
|
|
|
Types of non-ionizing radiation
|
-microwaves
-visible light |
|
|
Ionizing Radiation
-definition |
-radiation with enough energy to remove electrons from atoms, causing the atoms to become ionized
|
|
|
Types of ionizing radiation
|
-x-rays
-gamma rays |
|
|
Radioactive decay
-definition |
-the decay of an unstable atom, resulting in the emission of radiation (gamma rays)
|
|
|
Radioactive decay
-Types |
-alpha decay: 2 neutrons + 2 protons (He)
-beta decay: neutron change to proton and emits an electron (beta particle) -gamma decay: neutron and protons reconfigure within the nucleus (no change in N and P number) |
|
|
X-ray
-definition |
-a form of electromagnetic radiation traveling through space as a combination of electric and magnetic fields
|
|
|
Ways X-rays interact in different locations
|
-Waves when traveling through space (sine wave)
-Particles when traveling through matter |
|
|
Electromagnetic Radiation
-main characteristics |
-wavelength
-frequency -velocity |
|
|
Wavelength
-definition |
-distance between 2 wave peaks
|
|
|
Frequency
-definition |
-number of waves per unit time
|
|
|
Velocity
-definition |
-speed at which the electromagnetic ray moves
-speed of light |
|
|
How is energy related to the wavelength?
|

|
|
|
Basic energy unit for electromagnetic radiation
|
-electron volt (eV)
|
|
|
Electron volt (eV)
-definition |
-the energy of an electron accelerated by the potential difference of 1 volt
|
|
|
How much energy can result in ionization?
|
-15 eV
|
|
|
Electromagnetic Radiation
-properties |
-Can ionize atoms
-have no charge or mass -travel at the speed of light -invisible and cannot be felt -travel in a straight line -cannot be deflected by magnetic shields -penetrate matter to some degree -cause certain substances to fluoresce -expose photographic emulsions |
|
|
How are x-rays produced?
|
-electric current is passed through a filament (cathode)
-electrons boil off and form an electron cloud around the filament -a differential amperage is used to attract the electrons to the positively charged anode -high speed electrons strike metal target |
|
|
Energy of an X-ray is related to?
|
-velocity of the electron
|
|
|
Milliamperage (mA) control
-definition |
-controls the number of electrons produced in the electron cloud at the cathode
|
|
|
kVp control
-definition |
-controls the potential voltage difference at the anode to increase or decrease electron velocity
|
|
|
What is the effect of a higher kVp?
|
-higher velocity of electrons --> higher energy x-rays
|
|
|
Interactions that occur when electrons strike the anode
|
-collisional radiation
-radiative (predominant) |
|
|
What occurs in collisional radiation?
|
-an accelerated electron collides with an electron from an atom in the anode
-the electron from the anode is ejected leaving a positively charged "hole" in the atomic shell -an outer shell electron falls down into this hole and gives up some energy |
|
|
What occurs in radiative electron interactions?
|
-an oncoming electron from the cathode is slowed and changes direction due to an interaction with the nucleus of an atom in the anode
-the electron gives up energy as electromagnetic radiation -a range of energy is lost from the electron (0 keV - max kVp) |
|
|
kVp
-definition |
-the maximum voltage applied across the target-filament gap
|
|
|
Inverse Square law
-definition -equation |
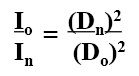
-the intensity of x-rays decreases with the square of the distance from the source
|
|
|
What is the inverse square law important for?
|
-focal film distance
-safety |
|
|
What are the 2 main ways the radiation interacts with matter?
|
-photoelectric effect
-compton scattering |
|
|
Describe the photoelectric effect
|
-an x-ray strikes the patient and is completely absorbed
-the absorbed photon ejects and electron (photoelectron) from a tissue atom and ionization occurs (similar to collisional radiation) -energy of the tissue emited x-ray is very low -low energy x-ray is absorbed in the patient and adds to the absorbed dose |
|
|
Why does the produced x-ray in the patient from photoelectric effect have such low energy?
|
-the atomic number of the tissue is lower than the atomic number of the anode
|
|
|
Factors affecting the probability of photoelectric effect occuring
|
-photon energy needs to exceed the electron binding energy
-an increase in the atomic number of the absorptive matter causes an increase in photoelectric effect -an increase in photon energy results in a decrease of the photoelectric effect |
|
|
What allows us to see contrast in radiographic images?
|
-the difference in atomic number of gas, fat, soft tissue, bone, and metal causing different photoelectric effects
|
|
|
Describe Compton Scattering
|
-incoming x-ray photon interacts with a peripheral shell electron of the patient
-the electron is ejected from the shell and a lower energy photon is scattered -the scattered electron can either cause more ionization or fog the film |
|
|
What is the cause for a majority of scattered radiation in diagnostic radiology?
|
-compton scattering
|
|
|
What does the probability of a compton interaction occurring depend on?
|
-the total number of electrons in the patient
-increased energy results in decreased scattering -independent of atomic number |
|
|
What is the result of compton scattering?
|
-decreased image contrast
-increased radiation dose for the image and the veterinary personnel |

